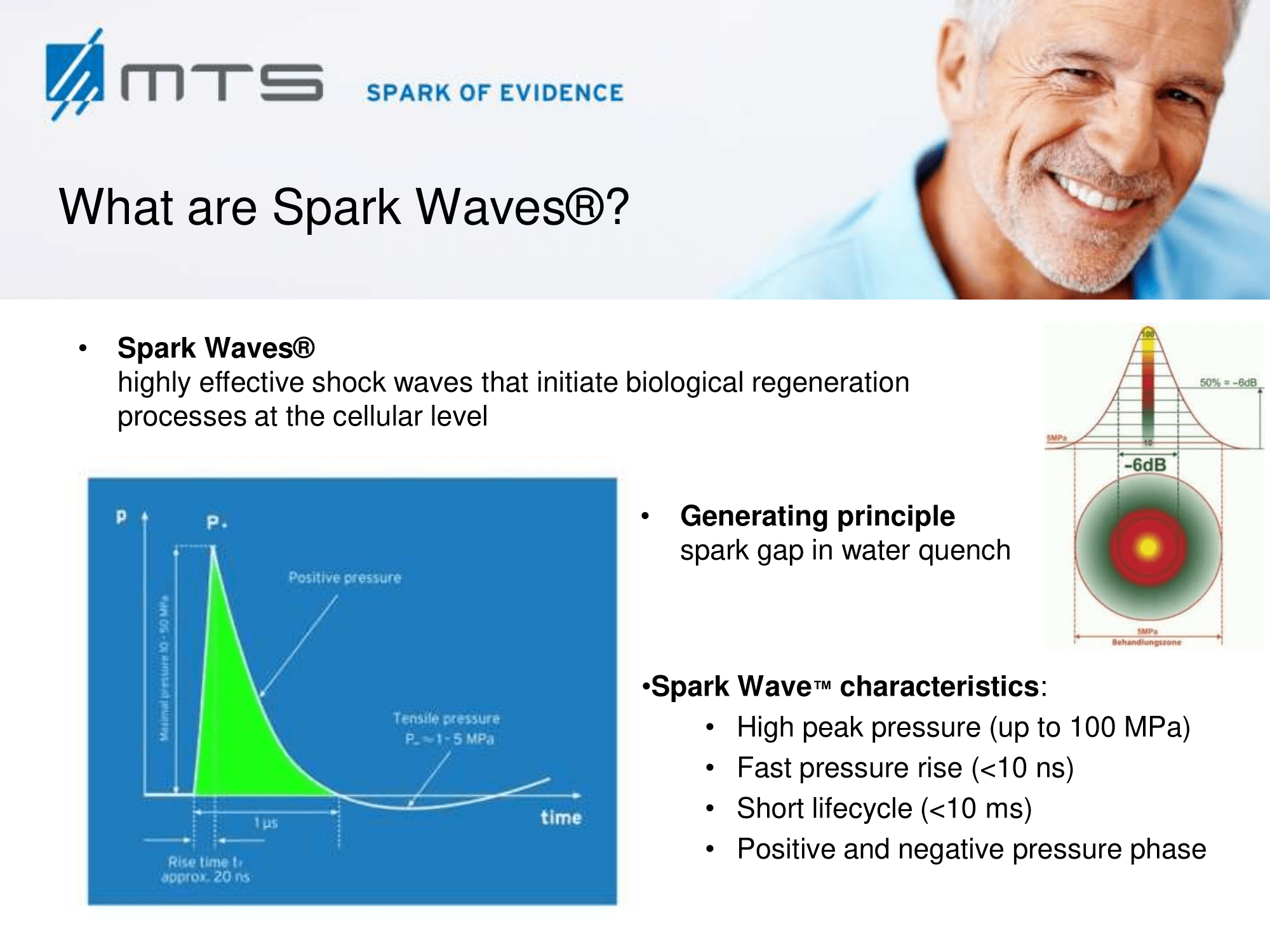
MTS Spark Waves® – a new method for the active promotion of wound healing
-
Introduction and overview
Acute wounds are caused exogenously by traumatic (e.g. mechanical, chemical, thermical) or iatrogenic (e.g. surgical) injury. In general, they are sharply limited and heal within short time without major complications.1 If there is no progress in healing after more than 4 weeks, they are referred to as chronic wounds, which arise from disturbed wound healing processes. In the majority of cases, the etiology of chronic wounds is multifaceted and includes local (e.g., venous or arterial insufficiency, atherosclerosis, infection, and local pressure) and systemic (e.g., immunological disorders, hypercholesterolemia, diabetes and nutritional status) factors.2 In contrast to acute wounds, the complex etiologies and prolonged treatment course have made the treatment of chronic wounds a challenge. 3 Chronic wound margins are imprecise and in the majority of cases they are infected. Chronic wounds have not completed the process of healing (restoring tissue loss and skin function), have not responded to initial treatment or persist despite appropriate care. 4,5 These wounds usually do not close without interferences and are sometimes resistant to healing interventions. The most common types of chronic wounds include venous leg ulcers (VLU), diabetic foot ulcers (DFU), pressure ulcers (PU), and arterial insufficiency ulcers (AIU), whereby especially elderly people are affected. 5 Poor response or failure to conservative treatments places a substantial burden on patients, their families, the healthcare system, and society in general. 6
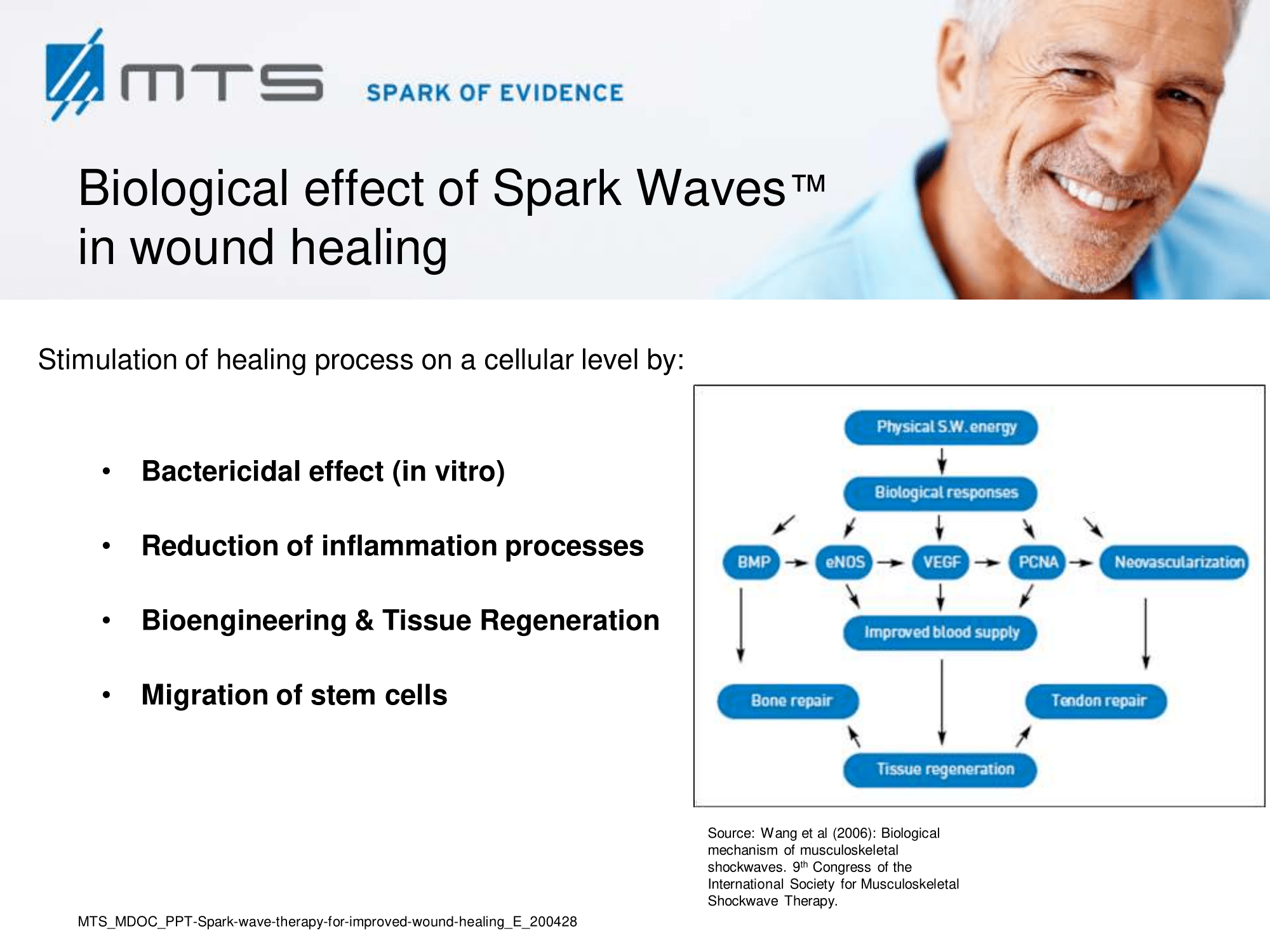
Although the process of healing is continuous, it may be arbitrarily divided into four phases: 1. coagulation and haemostasis; 2. inflammation; 3. proliferation / granulation; and 4. wound remodelling with scar tissue formation / epithelialization. 7 These broad varieties of cellular events are dependent on fluent cell-to-cell communication between signalling molecules as well as on an adequate nutrient supply. Therefore, a re-establishment of a functional vasculature, sufficient blood supply and the activation of cellular regeneration processes are crucial for wound healing in the traumatized tissue. 6,8 Extracorporeal Spark Wave Therapy represents an essential tool for regenerative medicine by promoting growth at different cellular levels.
-
Tissue regeneration through mechanotransduction by Spark Waves®
After regenerative effects of shock waves became evident, scientists step by step discovered numerous elements playing a role in healing processes. Although the precise biomolecular mechanisms of shock waves in tissues are still under investigation, it is most likely that the underlying principle of its effect can be based on a mechanical stimulation that becomes transformed into a cellular regeneration and growth-associated response. In recent years, the field of “mechanobiology” emerged in the scientific field and researchers began to analyze the cellular effects of physical stimuli and to elucidate this mechanotransduction by which cells and tissues adapt their molecular behaviour due to mechanical signals. 9 A quantity of mechano-sensitive molecules and cellular components that are involved in mechanotransductive biochemical responses have already been identified, such as stretch-activated ion channels, caveolae, integrins, cadherins, growth factor receptors, myosin motors, cytoskeletal filaments, nuclei, extracellular matrix, and numerous other structures and signaling molecules. 10 It has been shown, that extracellular vesicles are released by mechanical shear stress and transfer miRNAs between cells. 11,12
Animal model experiments and clinical studies illustrate that extracorporeal shock wave therapy (ESWT) for soft tissue wound healing can promote positive molecular and immunochemical reactions focused on improving blood flow microcirculation, activating anti-inflammatory response, and enhancing the tissue regeneration process. 1,9
-
Dermatological response to Spark Wave® application, research evidence
Inflammation constitutes the first response during the process of wound healing. Normally, it is thought to be beneficial for the organism since it limits continuation of tissue damage by clearance of pathogens and recruits cells and factors that ultimately lead to full tissue regeneration and functionality. 3 Inflammation shifts to chronicization and becomes pathologic if the healing process is disturbed, often due to age and persisting comorbidities of the patients. Macrophages represent key mediators during inflammation as they regulate the onset, the amplification and resolution of the response. The application of low energy shock waves lowered the pro-inflammatory and induced the anti-inflammatory profile in macrophages and altered the expression of cytokines and chemokines, like cyclophilin A, interleukins-6, -10 and -1β and of immune-receptors like the TLR3 (toll-like receptor 3) and other players. 14–18 Furthermore, in in vivo studies, shock waves reduced leucocyte and macrophage infiltration into isograft tissue and decreased the corresponding macrophage-derived inflammatory protein response (MIP-1 α and β), pointing on an anti-inflammatory mechanism of shock waves. 19,20
Infection of chronic wounds is a major challenge during the treatment. Microbial colonization maintains the inflammation and impairs the process of healing. Shock waves have a bactericidal effect and are able to reduce the bacterial burden of the affected tissue. 22–25 A further beneficial aspect of SWT is that it increases the local number of microvessels and improves the systemic delivery of antibiotics to the infected wound.
Apoptosis and necrosis are known to have a high impact on regenerating tissue. Apoptotic cells can produce harmful signals that have a profound influence on neighboring cells and tissues linked to numerous pathologies. Necrotic cells release cellular contents and factors into the extracellular space which cause inflammation and further cell death. 26 In several studies it was demonstrated, that shock wave treatment decreases cellular apoptosis and can reduce necrosis in wounds. 16,27–29
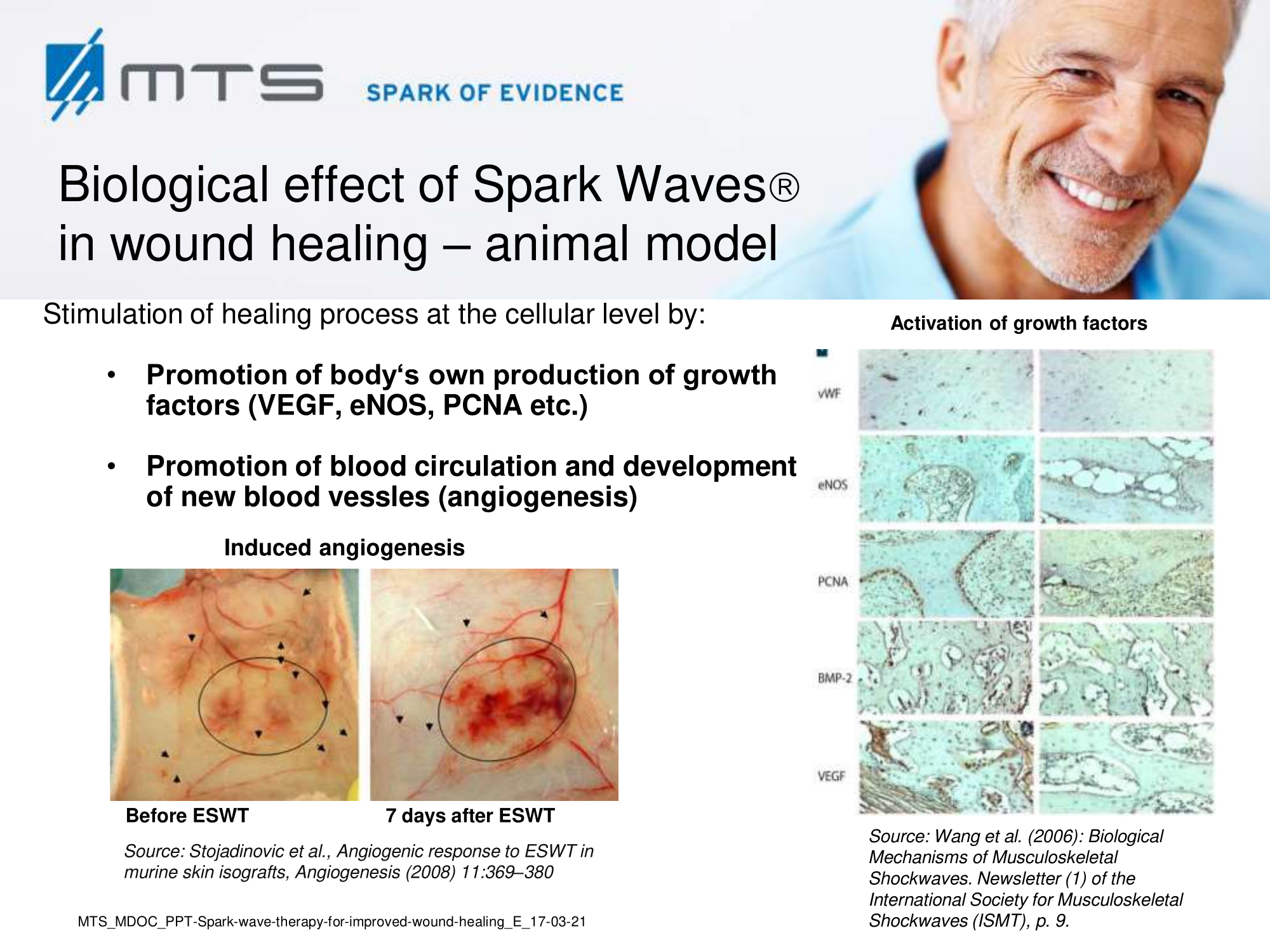
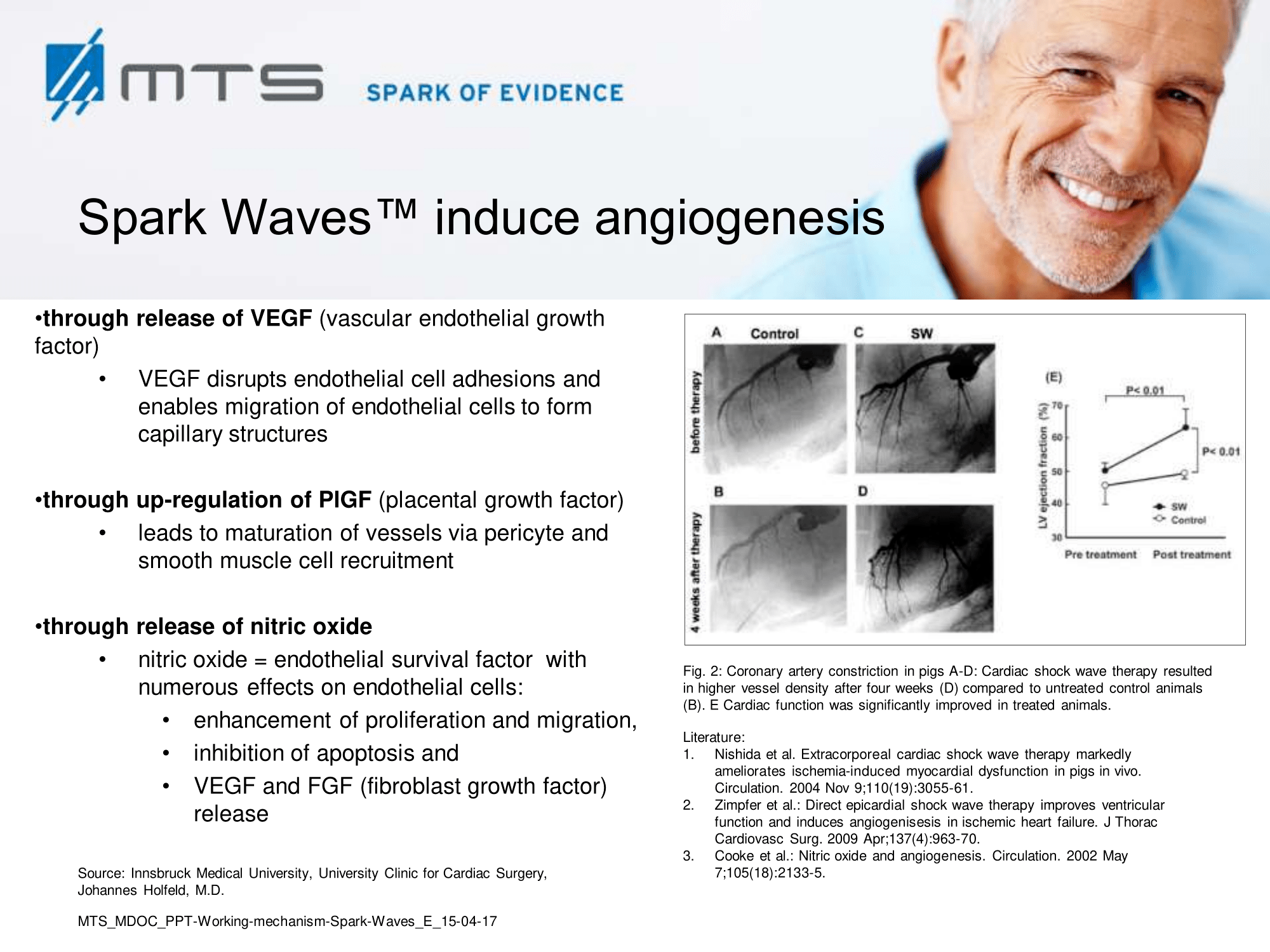
Tissue remodelling and neovascularization: Re-establishment of a functional vasculature is the most critical determinant of restored tissue structure and function in wound healing, which largely occurs via angiogenesis, endothelial sprouting and vasculogenesis. 6,8,17,30–32 As the wound starts to heal, cells proliferate and build granulation tissue which is strongly permeated by capillaries. Later on, in the course of epithelialization, scar tissue is formed. Shock wave treatment (SWT) was shown to accelerate granulation and reepithelialization, and to reduce scar formation. 17,33–36 SWT increased the overall blood circulation of affected areas and stimulated cutaneous and muscular microcirculation. 28,29,37–39 In this respect, shock waves proved to be highly effective and beneficial, since treatment induced recruitment of endothelial progenitor cells and the expression of angiogenic factors like VEGF or TGF-β. 40–42 Nitric oxide (NO), a potent vasodilator, is another key player of shock wave-improved local blood flow and an important mediator of angiogenesis in the wounded area. NO became elevated upon SWT and enhanced tissue perfusion, partially due to the increased performance of nitric oxide synthase (NOS). 3,43,44 The primary mediator of angiogenic signaling -the vascular endothelial growth factor (VEGF)- and its corresponding receptor VEGF-R2, were also shown to be up-regulated upon ESWT treatment in numerous studies. 45–47 VEGF stimulated multiple components of the angiogenic cascade, capillary growth and promoted epithelialization and collagen deposition in the wound. 48,49
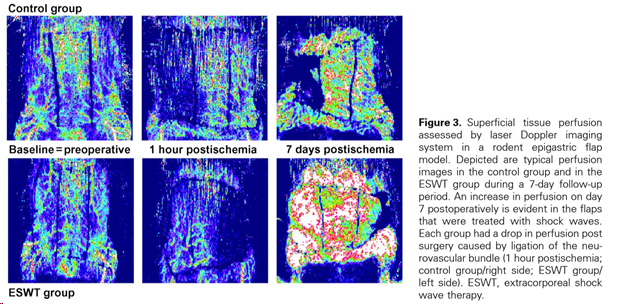
Enhanced tissue perfusion upon shock wave therapy
(Figure and caption 1)
Furthermore, it was reported that ESWT has a positive effect on the expression of other important growth factors like BNDF (brain-derived neurotrophic factor), BMP (bone morphogenetic protein) and TGF-β (transforming growth factor), FGF-2 (fibroblast growth factor), IGF-1 (insulin growth factor) and PCNA (proliferating cell nuclear antigen). 50–55 Hence, SWT strongly induces cascades of cell-proliferation and tissue re-growth. 28 Especially the activation of TGF-β1 and collagen type I and III, which are main factors involved in repair processes of connective tissues, confirmed a beneficial role of SWT in regeneration of the skin. 51 It stimulated proliferation and recruitment of fibroblasts by boosting extracellular matrix metabolism and connective tissue interaction. 19,41,51 Moreover, SWT led to recruitment, proliferation and differentiation of mesenchymal and penile progenitor cells. 56–58 The recent “bench to bedside”-study by Aschermann et al. demonstrated, that extracorporeal shock waves activate morphological changes and increase cell migration of keratinocytes. Cell-cycle regulatory genes were up-regulated and proliferation in fibroblasts was induced. This was accompanied by secretion of pro-inflammatory cytokines from keratinocytes, which are known to drive wound healing, and a pro-angiogenic activity of endothelial cells. 59 They demonstrated improved wound healing upon SWT in an open-label, single-arm study in patients with therapy-refractory chronic leg ulcers. 60 The toll-like receptors (TLRs), in addition to their established roles in the immune defence system, have emerging roles in controlling homeostasis, injury and wound repair. The dsRNA-sensing receptor, TLR3, has been particularly implicated in such processes in several different tissues including the skin, intestine and liver, as well as in the control of reparative mechanisms in the brain, heart and kidneys, following ischemia reperfusion injury. 61 In 2013 and 2017, Hohlfeld et al. showed that SWT induced angiogenesis in endothelial cells specifically by stimulation of TLR3 signaling. 18,62
Nociception and pain reduction are another important part of wound care. Several multidisciplinary studies analyzed the role of ESWT in this respect and found that it has an analgesic effect and is able to suppress and relief pain which would be certainly beneficial for patients suffering from severe wounds and painful scars 3,6,27,36,63–69.
-
Biological mechanisms of shock wave therapy at a glance
- Growth factor activation:
- Nitric oxide (NO)
- Fibroblast growth factor (FGF)
- Transforming growth factor (TGF)
- Insulin-like growth factor-1 (IGF-1)
- Plateled-derived growth factor (PDGF)
- Vascular endothelial growth factor (VEGF)
- Proliferating cell nuclear antigen (PCNA)
- Increased coutanous microcirculation and dermal metabolism
- Fibroblastic proliferation
- Stem cell migration and differentiation
- Enhanced tissue perfusion
- Increased collagen deposition
- Enhanced granulation tissue formation
- Reduction of necrotic fibrin tissue
- Anti-inflammatory action
- Accelerated wound closure and reepithelialization
-
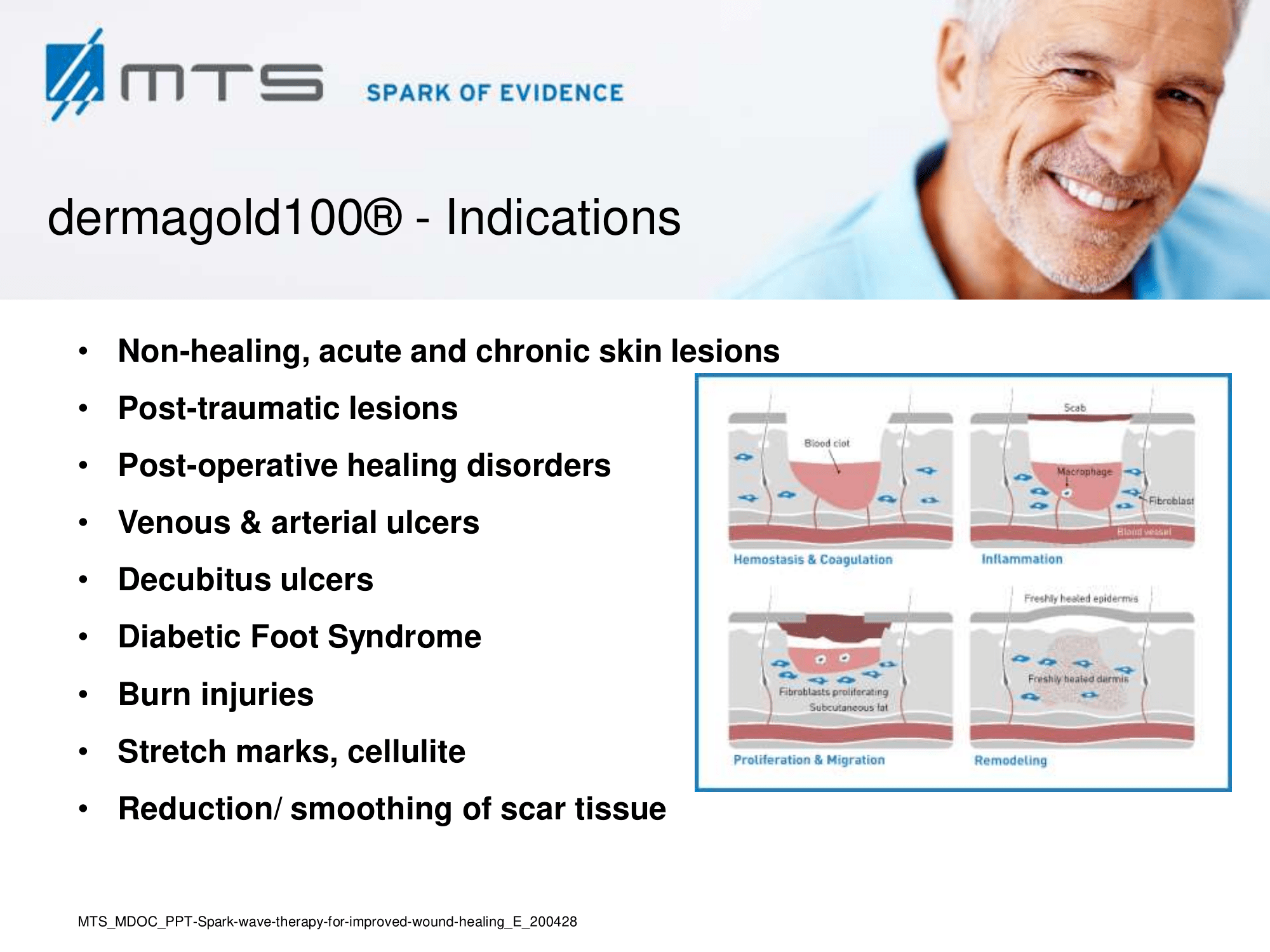
Wound care indications of Spark Wave® Therapy, clinical evidence
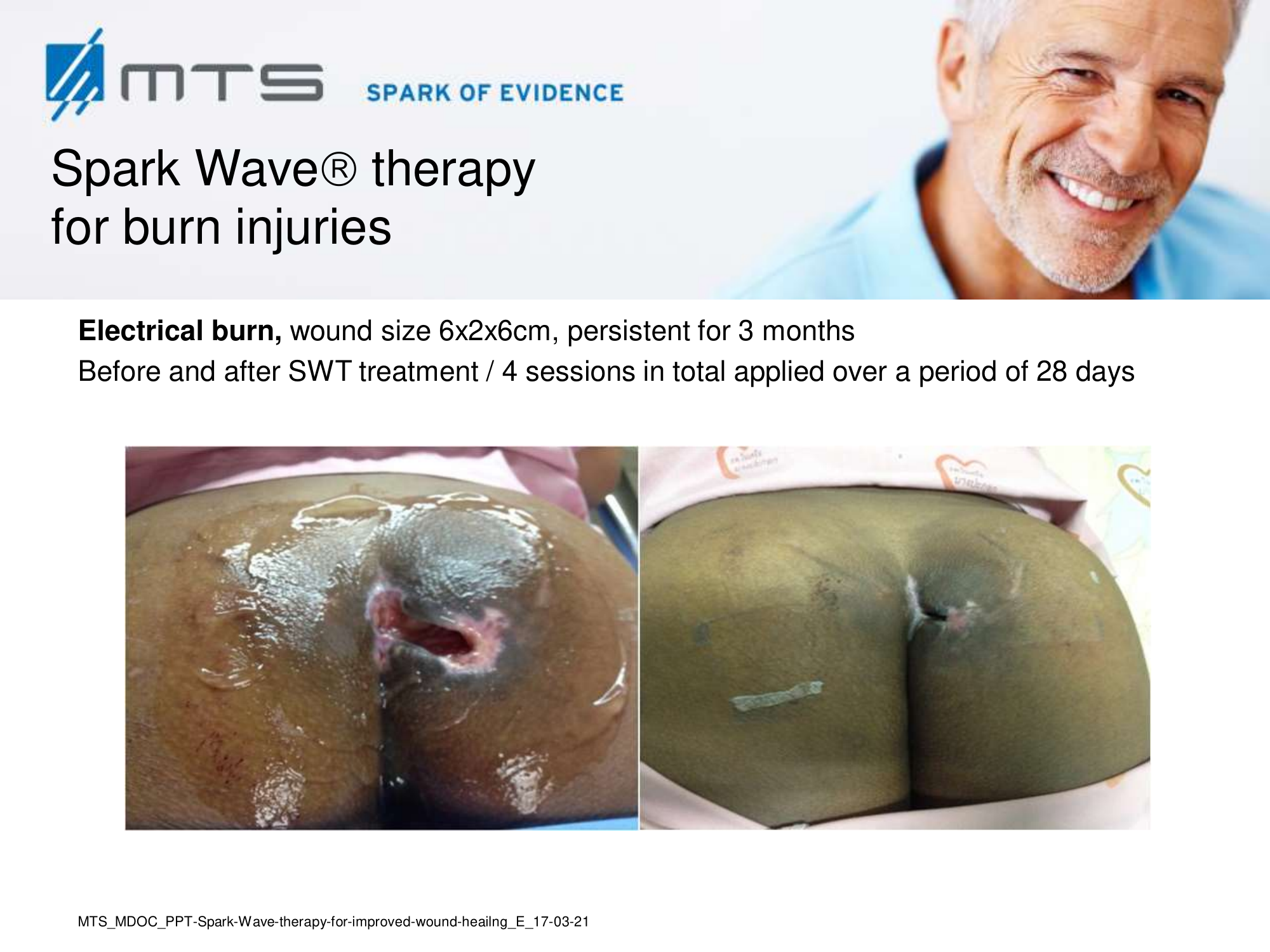
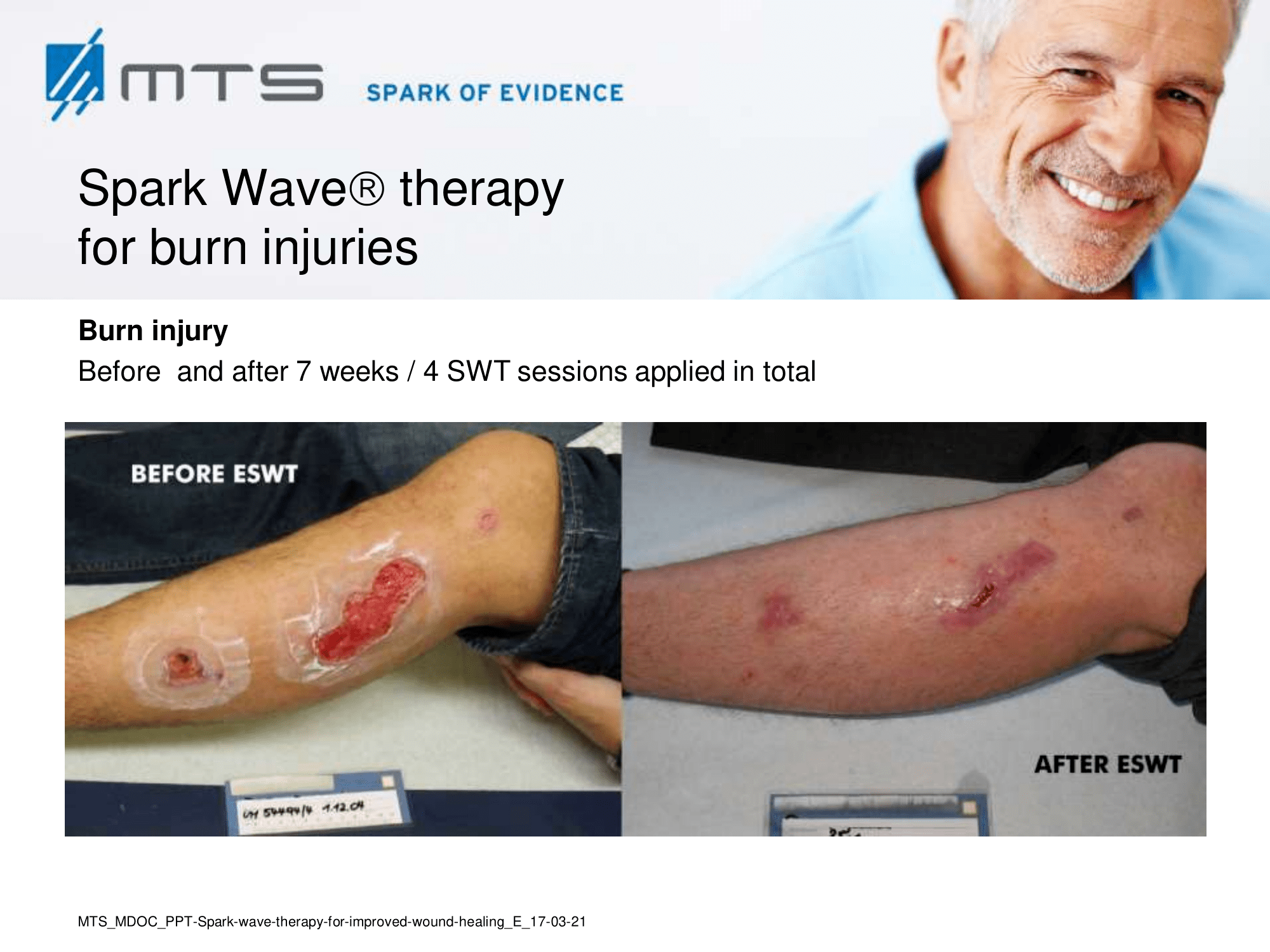
- Burns and scars
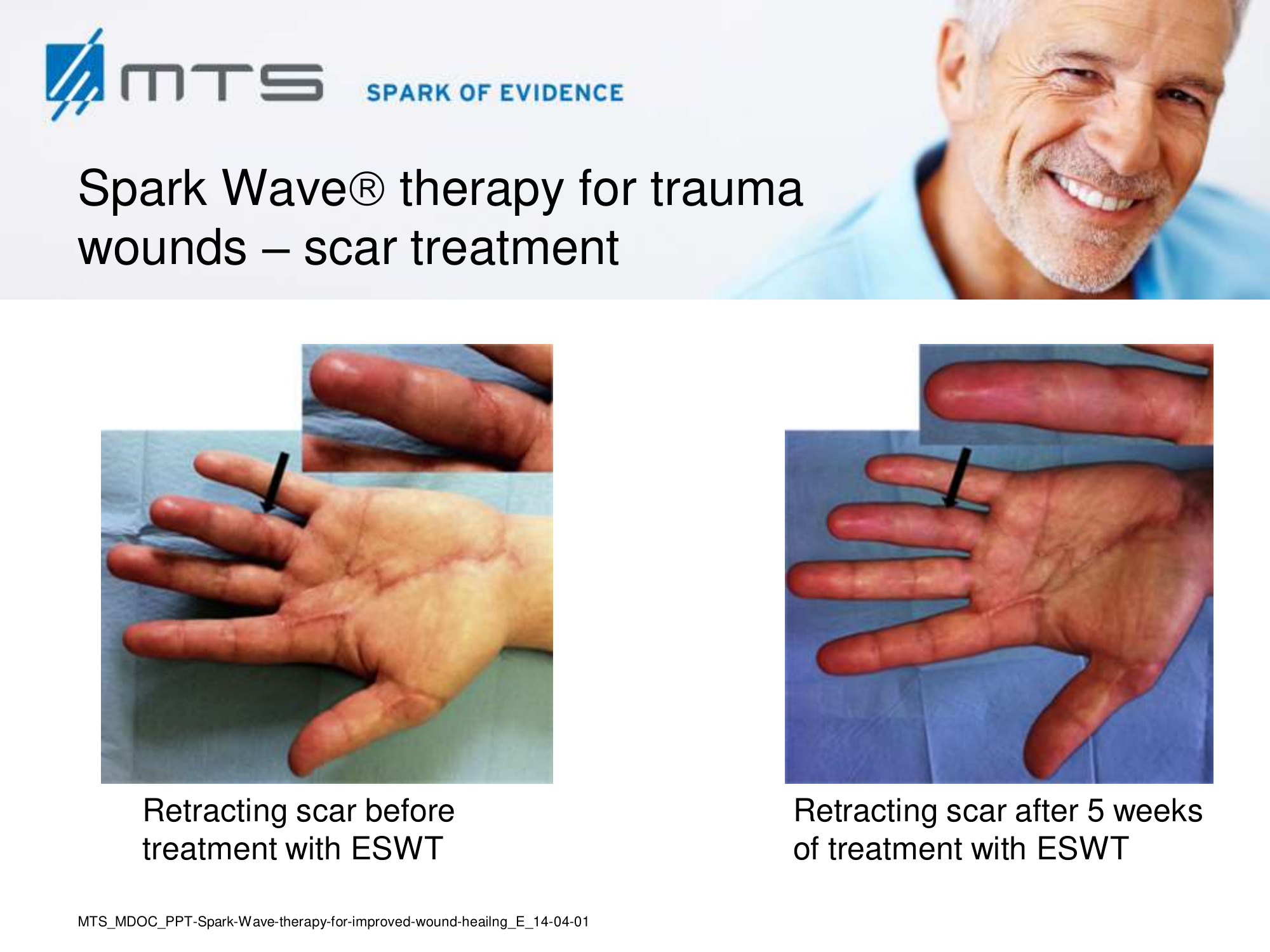
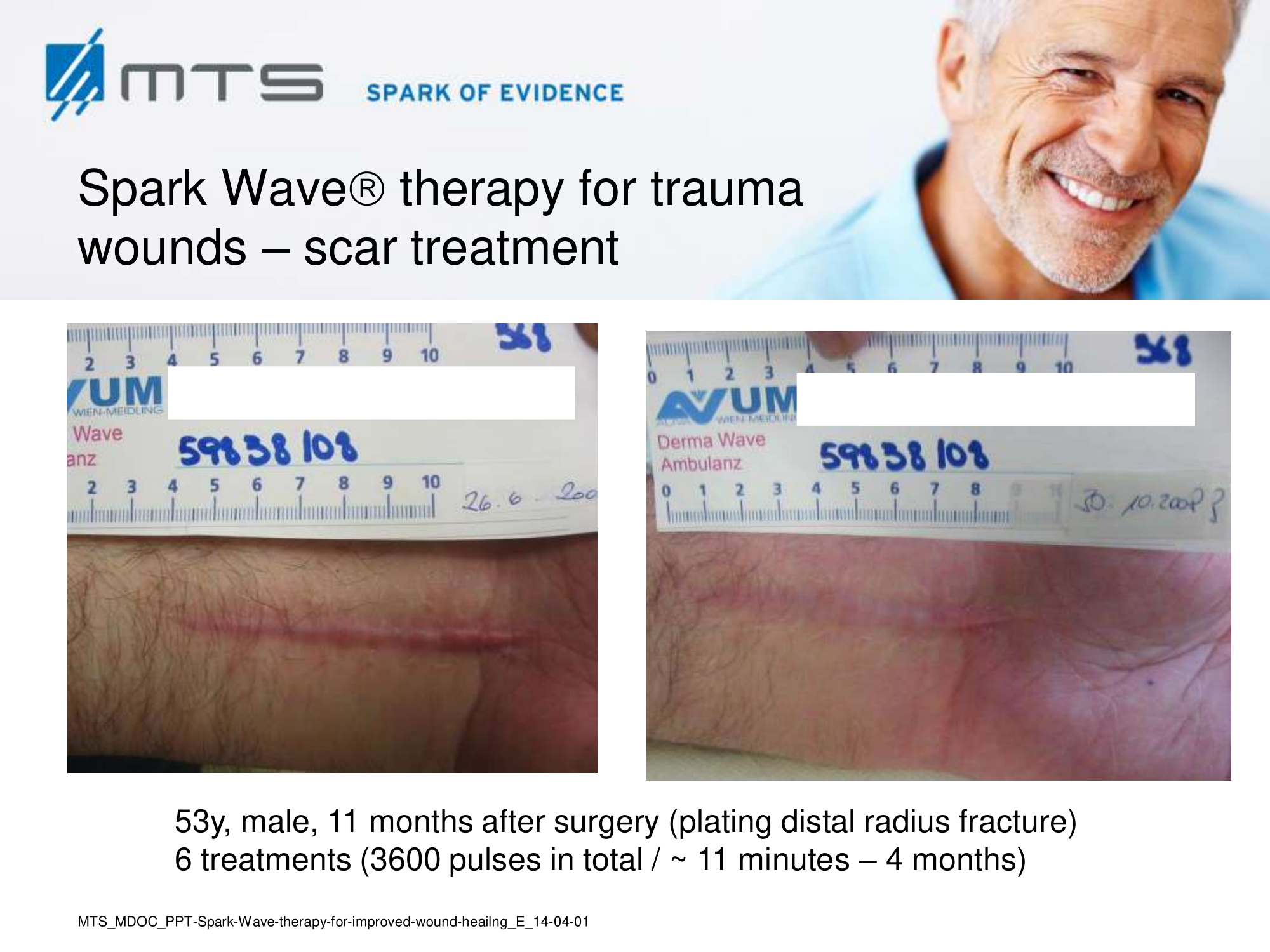
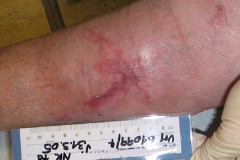
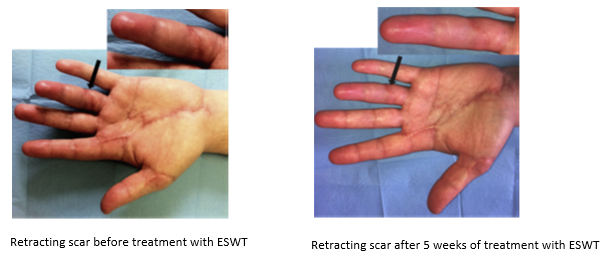
Randomized controlled trials show that ESWT significantly accelerates reepithelialization of second-degree burns, reduces scar pain and burn-associated pruritus. 35,63,70 Treatment of keloid scars with ESWT resulted in significant decreases in collagen fibers and increases in MMP-13 enzyme and could decrease the need of surgery in the treatment of deep partial/full thickness burns, skin grafting could be prevented. 71–73 ESWT significantly improved scar clinical appearance, hand mobility and subjective pain of retracting hand scars. Histopathological examination revealed significant increase in dermal fibroblasts, neoangiogenic response and type-I collagen concentration. 36 ESWT is a feasible and cost-effective treatment in the management of postburn pathologic scars. 34
In-depth basic research shows, that the successful treatment of burns and scars with ESWT is due to its anti-scarring, anti-inflammatory, pro-angiogenic effect. 20,31,74–77
 |
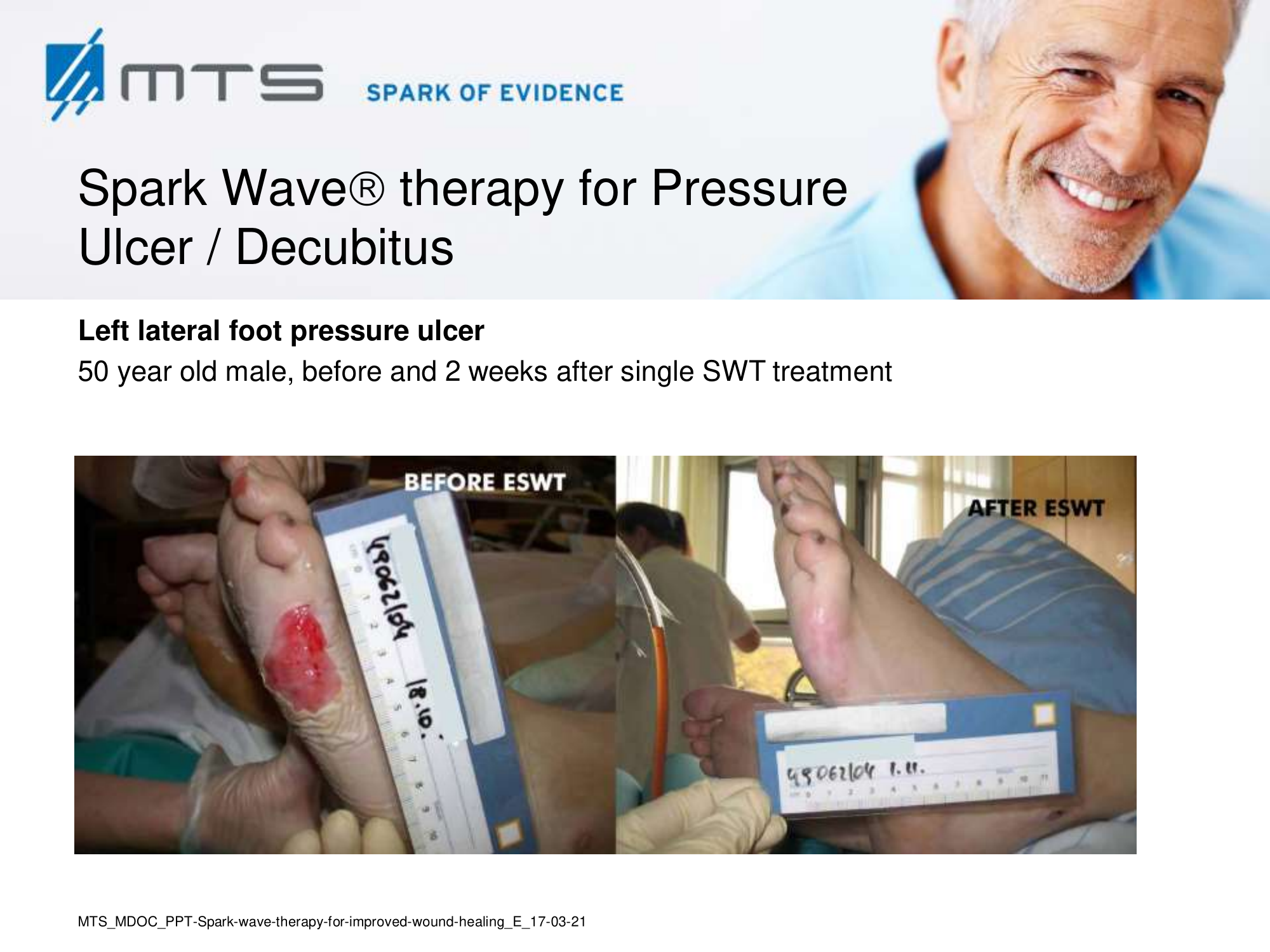
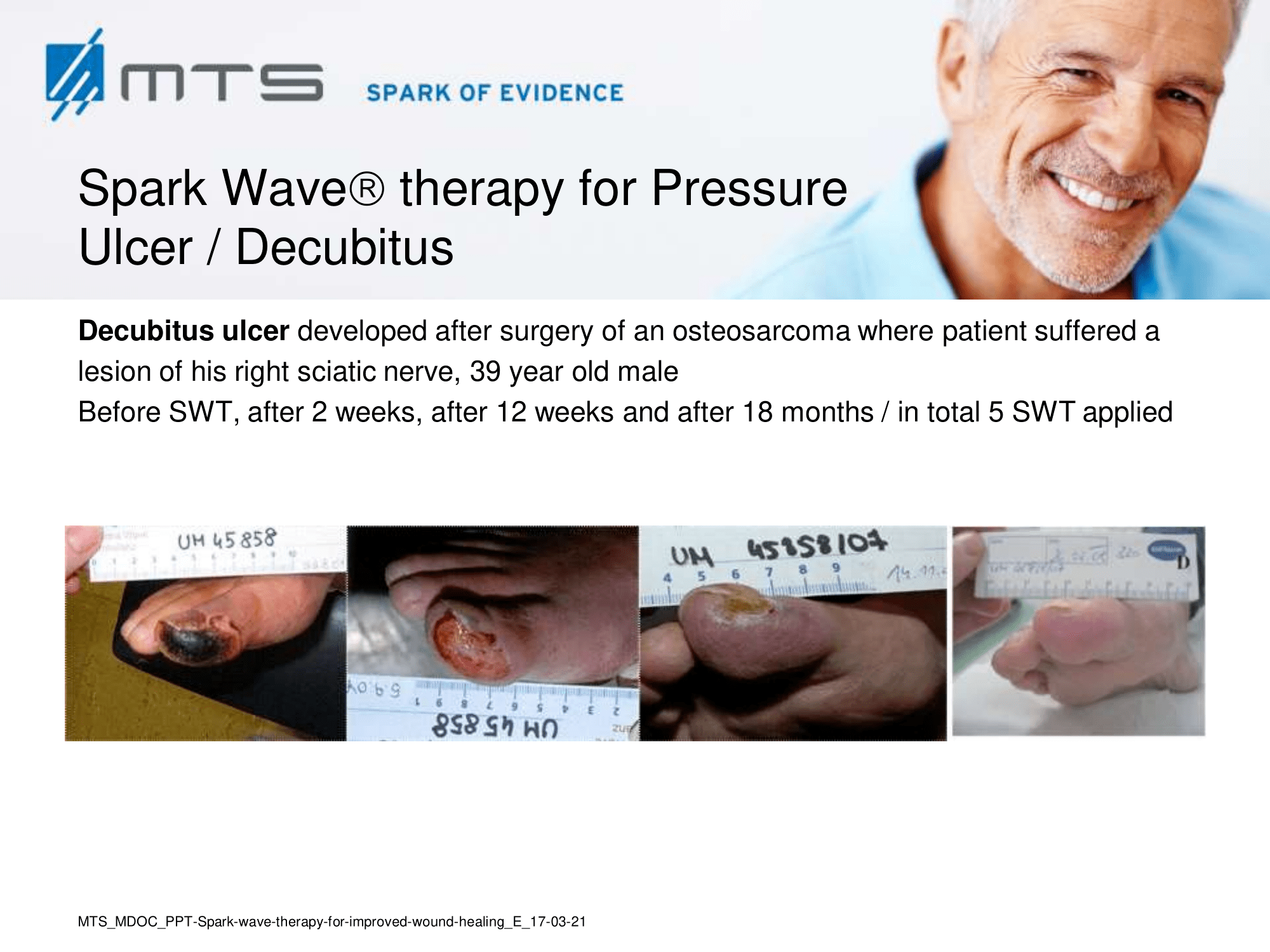
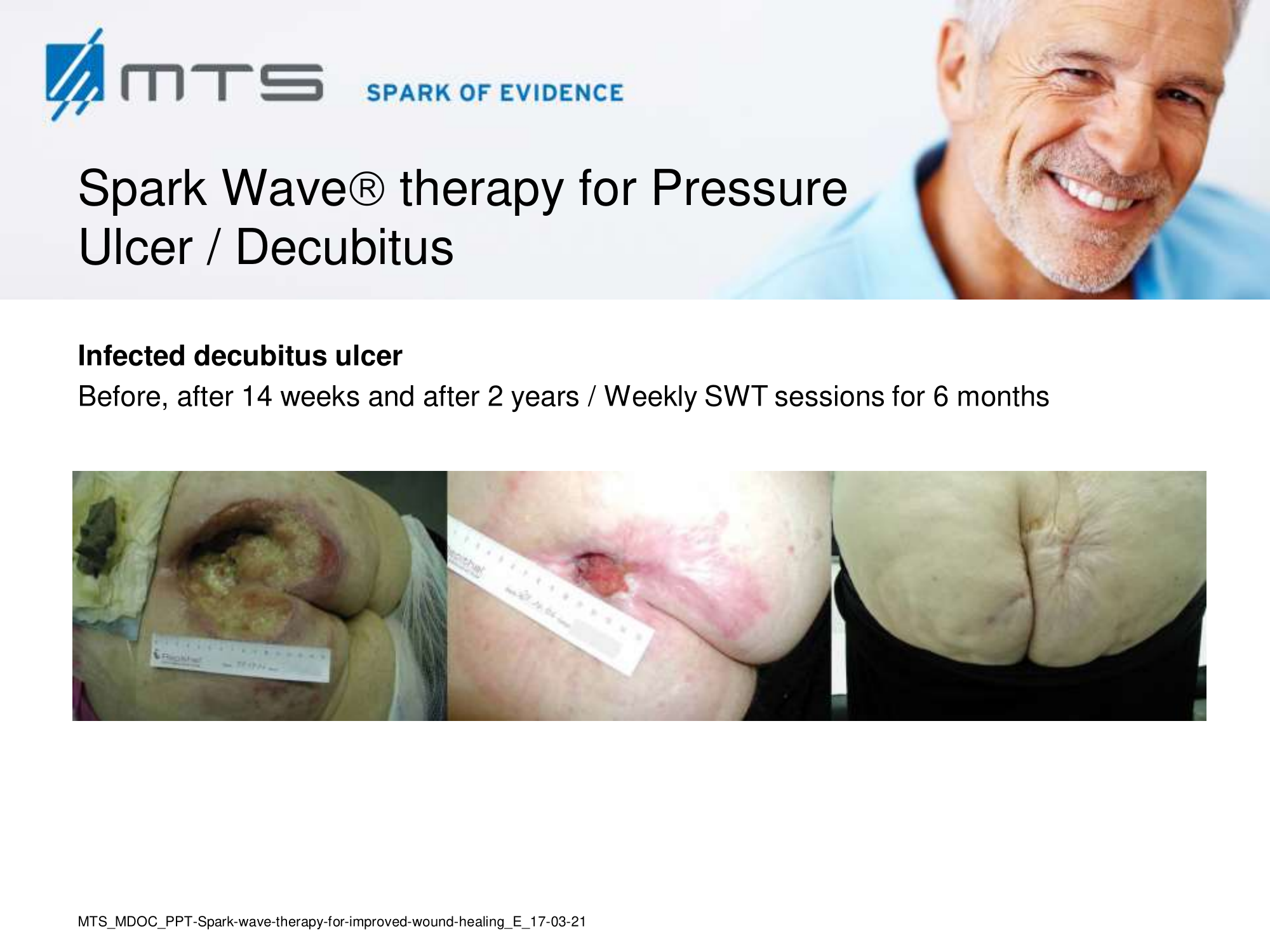
Non-healing ulcers, decubitus ulceration
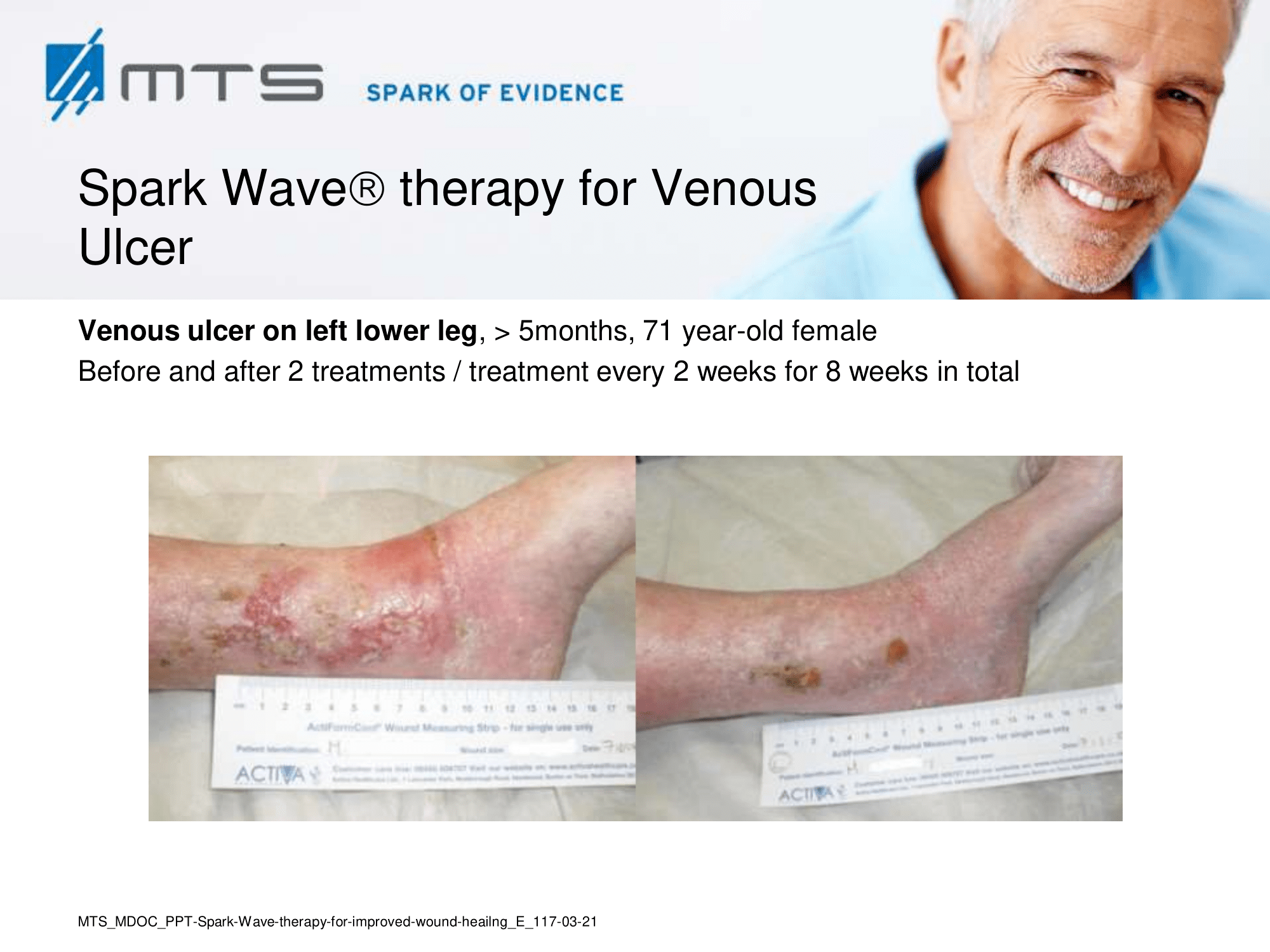
In case of difficult-to-treat lower limb ulcers, ESWT is a complication-free, effective tool that accelerates wound closure by stimulating angiogenic factor release from endothelial cells and fibroblasts. Several clinical studies demonstrated that extracorporal shock waves activate migration, proliferation and inflammatory pathways in fibroblasts and keratinocytes, and significantly improve wound healing in therapy-refractory chronic leg ulcers. ESWT initiates healing of static chronic ulcers in people with complex neurological disabilities. 60,78–80
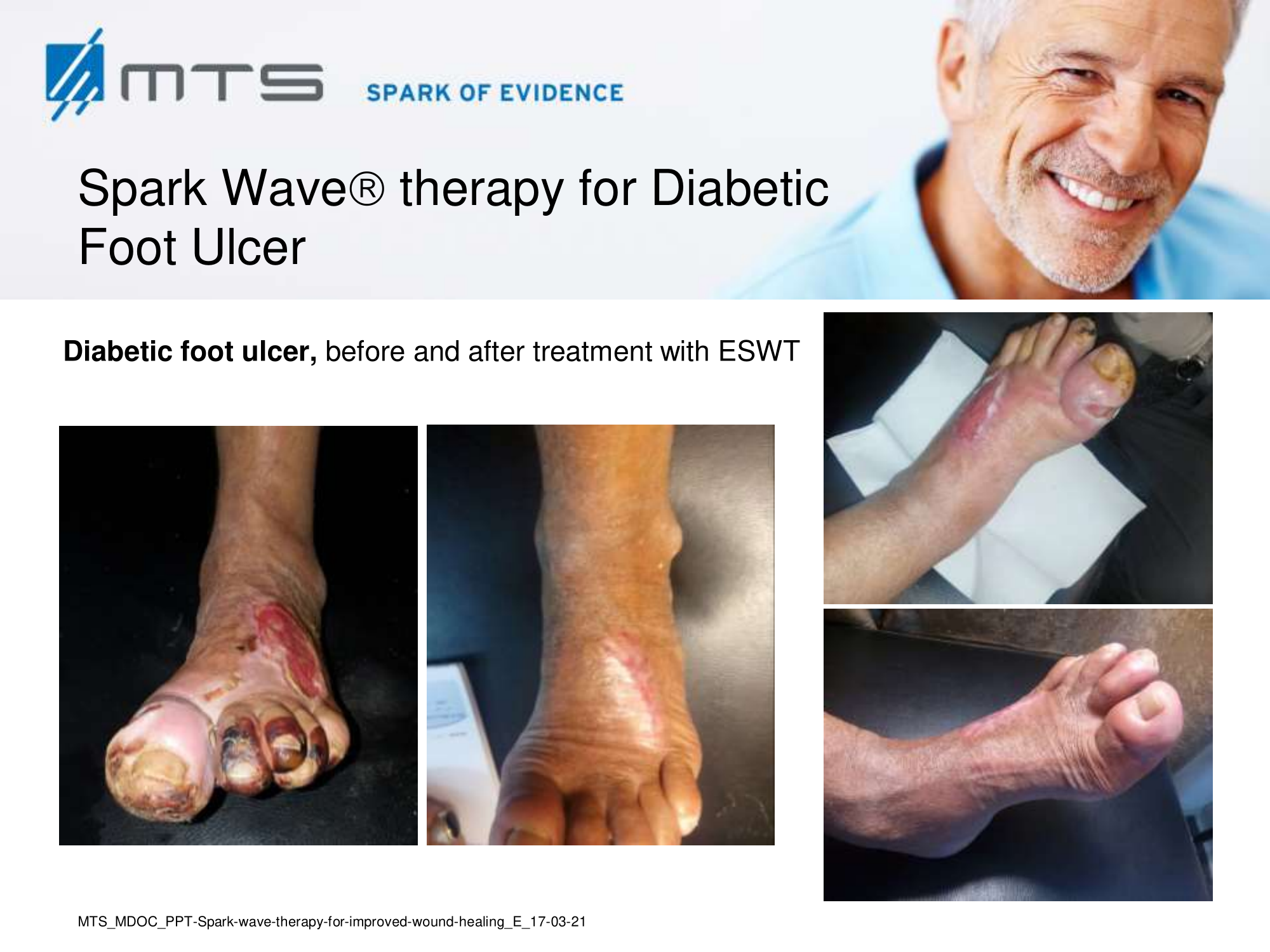
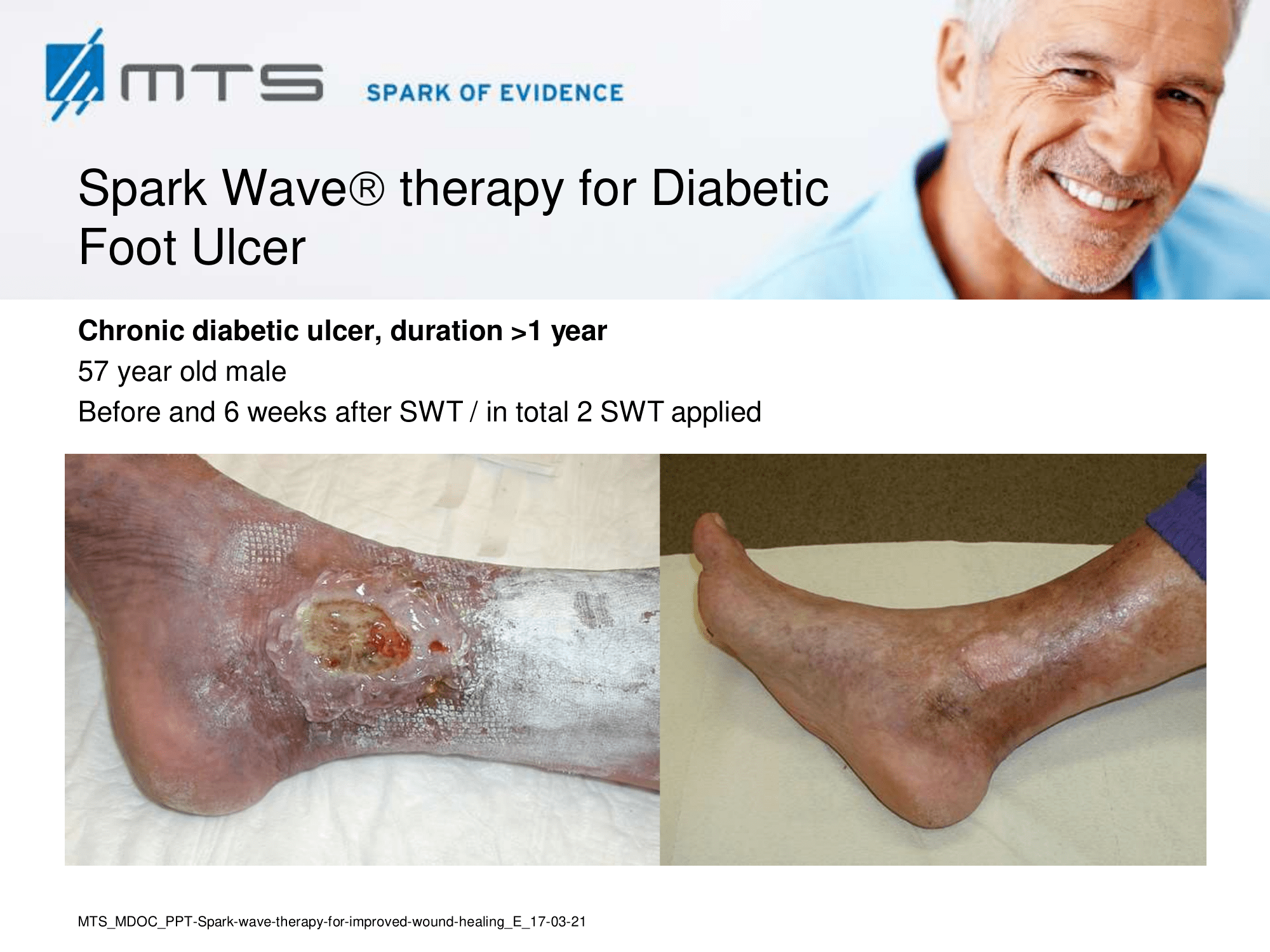
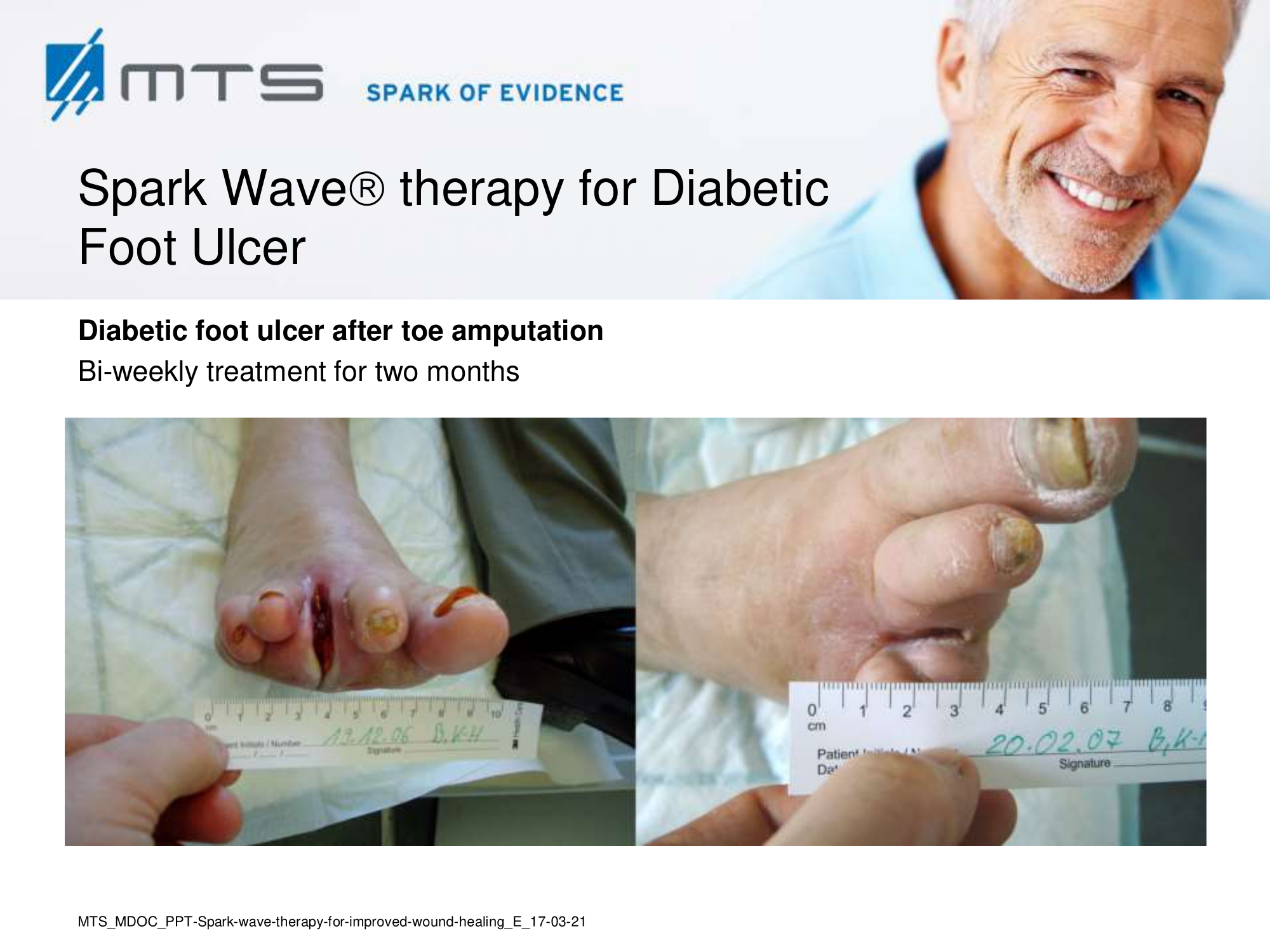
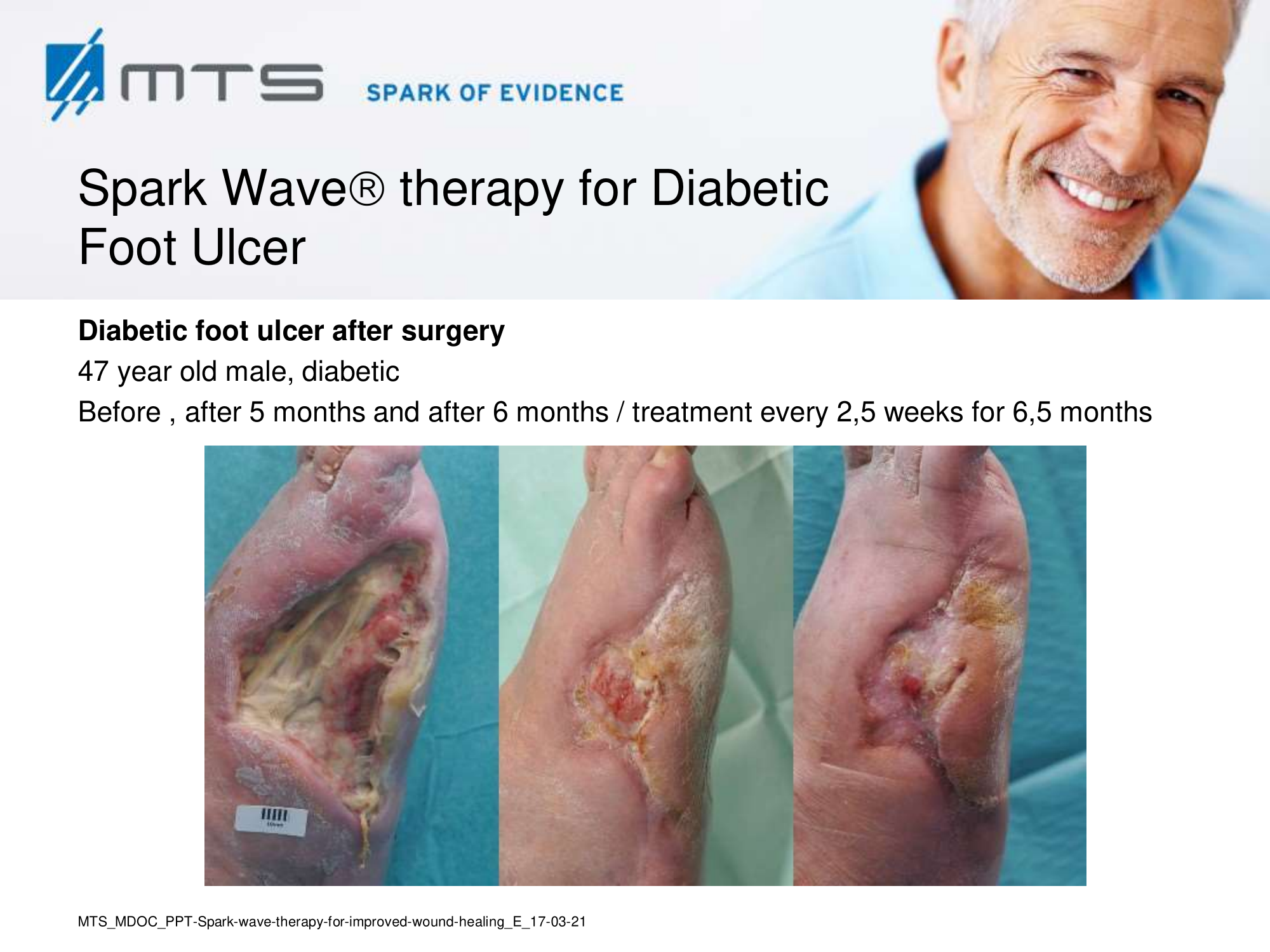
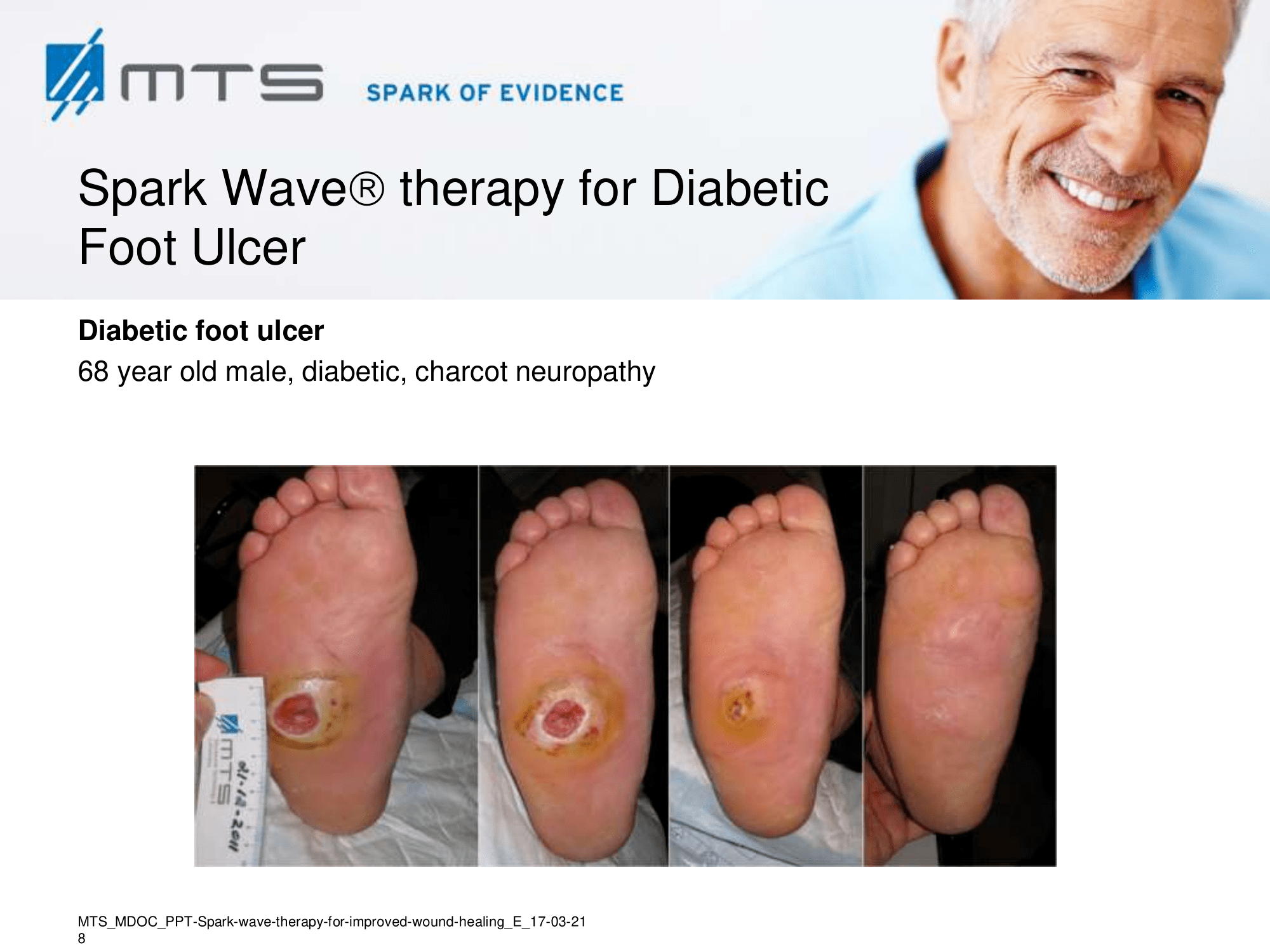
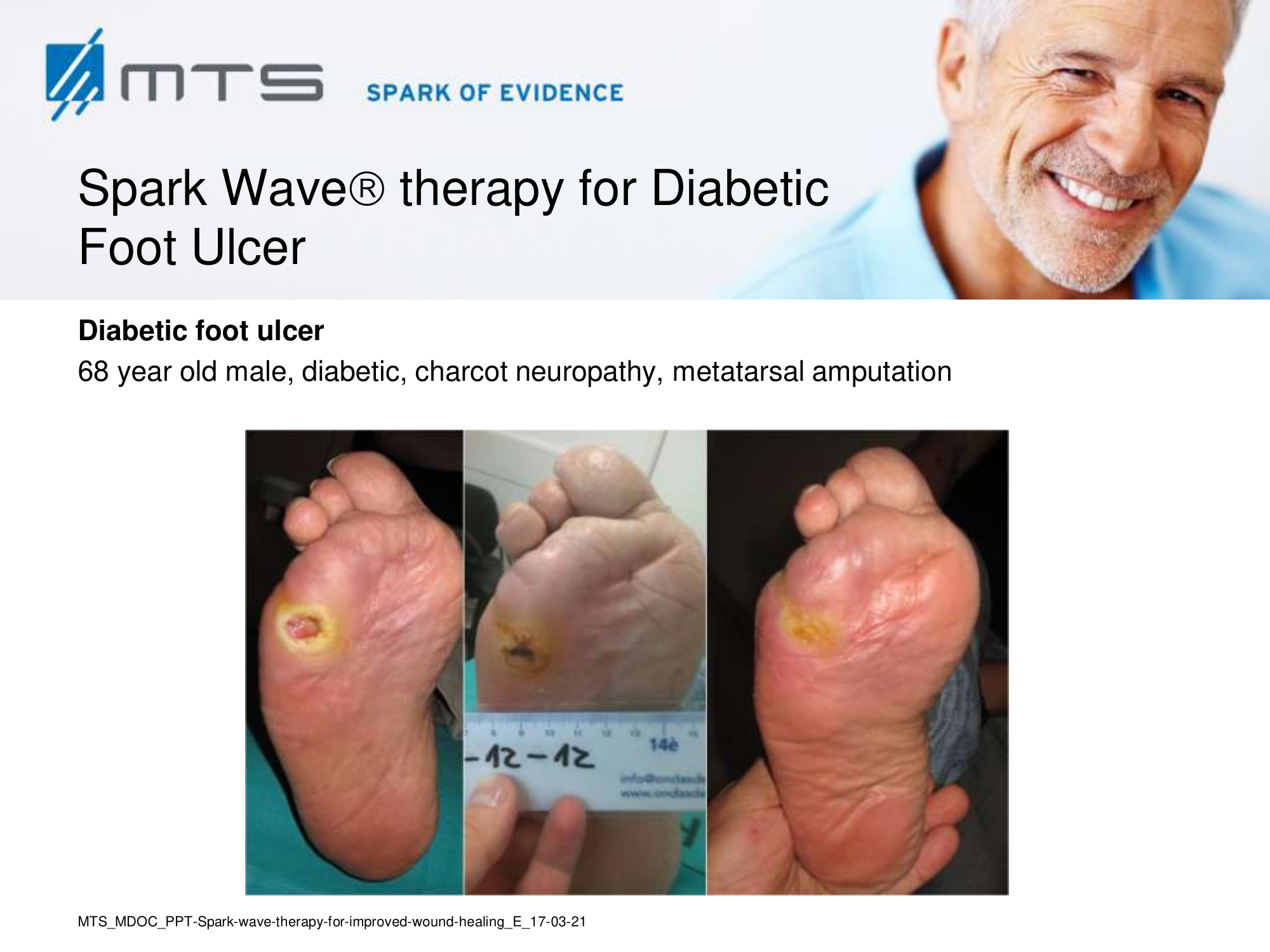
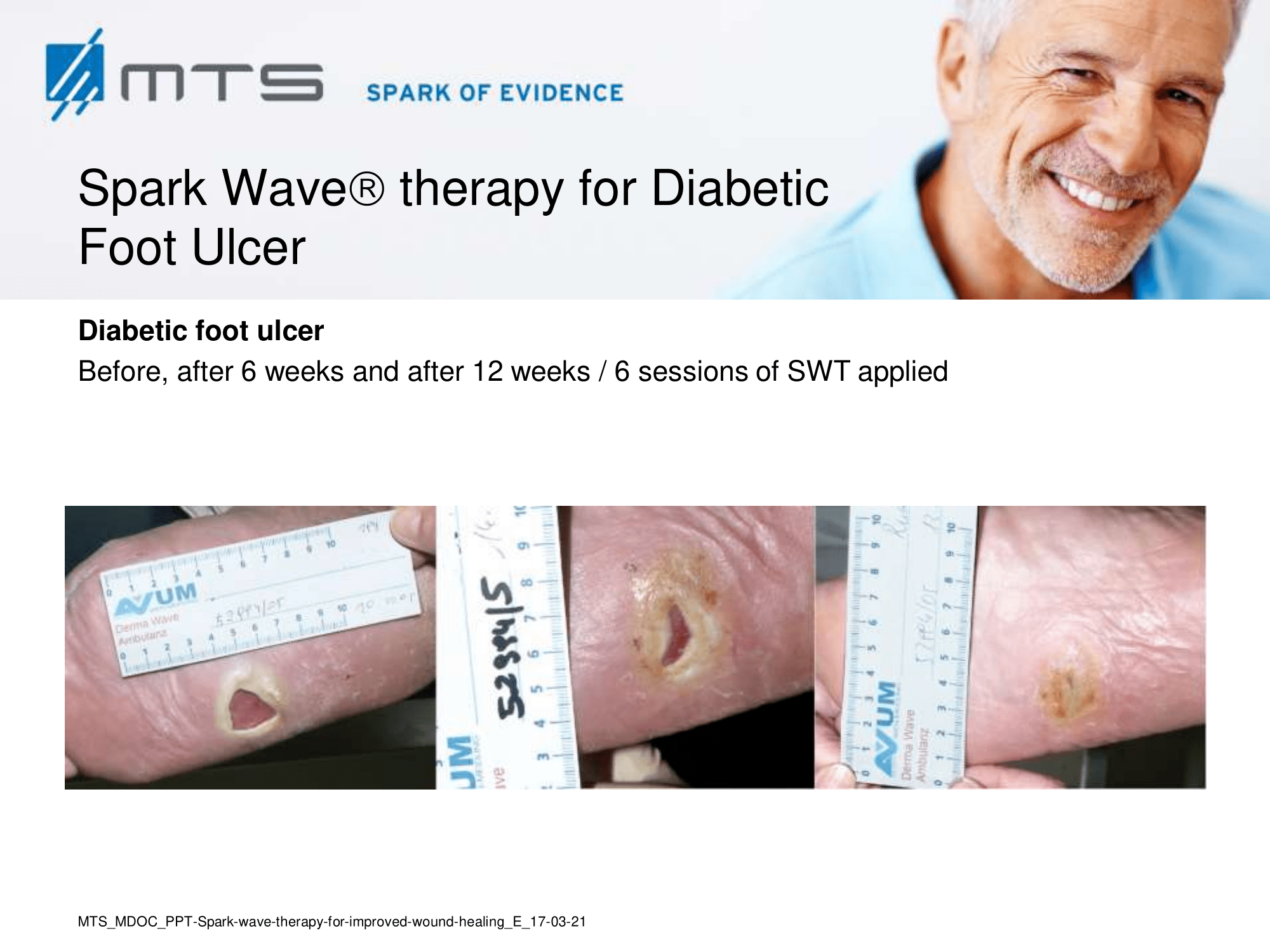
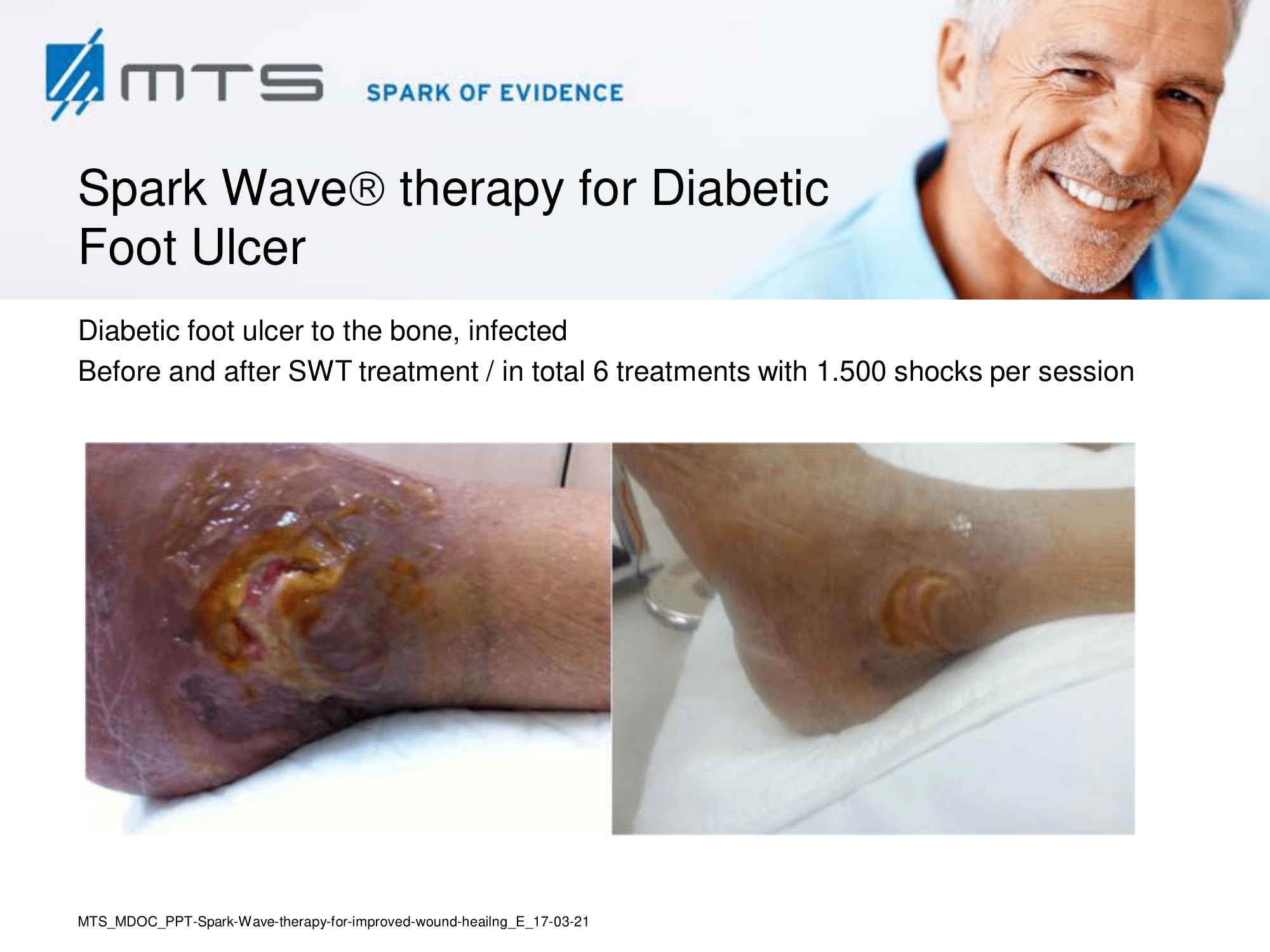
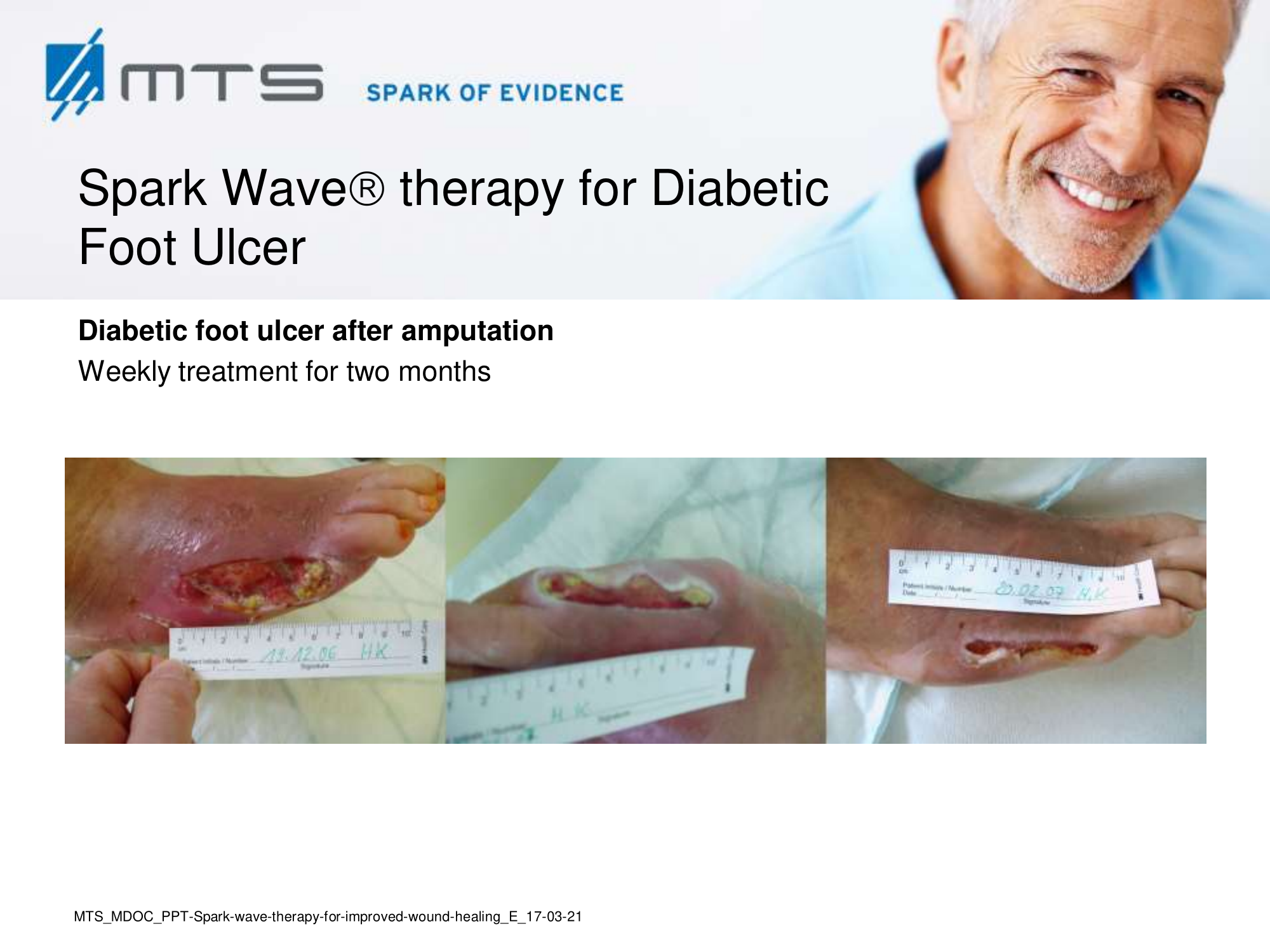
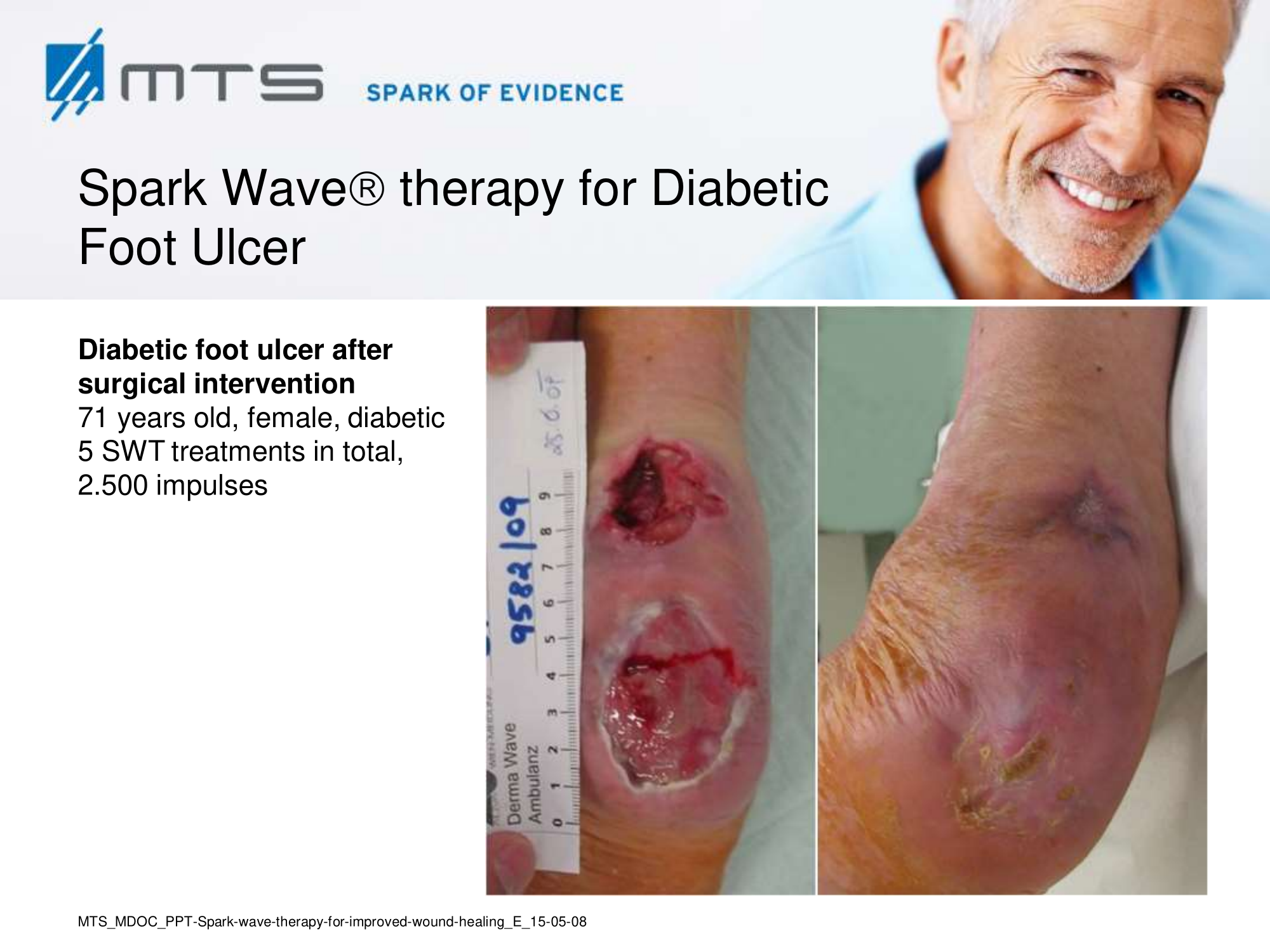
- Chronic Diabetic foot ulcers
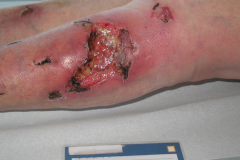
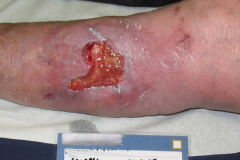
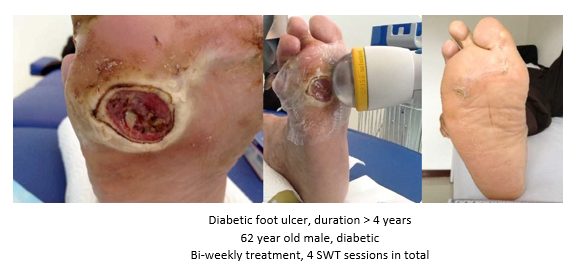
The pathological characteristics of DFUs are an impaired neovascularization, a prolonged inflammatory phase and fibroblast dysfunction. Three randomized controlled clinical trials have been performed, showing that ESWT-treated DFUs have reduced wound size and healing time, which makes it a useful adjunct treatment. ESWT groups showed increased reepithelialization and tissue oxygenation. 81–84 Wang et al. reported that ESWT leads to significant improvement of blood flow perfusion rate and cell activity, leading to angiogenesis and tissue regeneration, even superior to hyperbaric oxygen therapy (HBOT). 25,33,85,86
Various basic research studies revealed the underlying molecular mechanisms which lead to improved wound healing in diabetic animal models. ESWT lead to significantly reduced wound size by lowering the topical pro-inflammatory reaction, enhancing pro-angiogenic response factors (VEGF, eNOS, PCNA), greater number of fibroblasts, up-regulated TGF-ß1 expression, enhanced tissue granulation and neocollagenesis. 38,87–91
 |
(Figure and caption 3)
- Calcinosis cutis, systemic sclerosis
In recent years, ESWT becomes applicable during the treatment of complex skin disorders like calcinosis cutis and systemic sclerosis with successful outcome. Here, shock wave therapy also reduced wound pain and promoted epithelialization and healing. 69,92–96
- Skin transplant
Spark Wave Therapy significantly accelerates donor site epithelialization after application of a single defocused shock wave treatment immediately after skin graft harvest as demonstrated in a recent randomized, controlled trial. 97 Substantial animal research has been performed. It was shown that ESWT enhances skin flap survial, protects against ischemia reperfusion injury and minimizes tissue necrosis by promoting tissue revascularization. Improved blood flow though angiogenesis is mediated by shock wave-activated expression of NO and VEGF. Furthermore, ESWT suppresses tissue pro-inflammation. 17,19,29,98–111
- Fibromatosis
ESWT can be also utilized to treat excresences of the connective tissue or capsular fibrosis after breast implants. Application of shock waves reduced pain in painful plantar fibromatosis. 112 In animal experiments, ESWT decelerated capsule formation, degraded fibrotic tissue, decreased scar formation and collagen deposition. 113–115
- Lymphedema
Lymphedema may be inherited (primary) or caused by injury to the lymphatic vessels after surgery or radiation (secondary). Shock wave treatment stimulates the cell metabolism and increases blood and lymph circulation. Studies, investigating the clinical effect of shock waves on lymphedema after breast cancer treatment, report reduced lymphedema and a marked improvement of the functional statement and quality of life of patients. 116–120
- Leprosy
Patients suffering from Hansen`s disease (Leprosy) develop chronic ulcers during the course of the disease due to a neuropathic, hypovascular, hyporegenerative infectious background. In a first blinded, randomized clinical case control trial, Leal et al. (2014) investigated the effect of wide focused Spark Wave Therapy in patients with Leprosy. ESWT significantly improved wound closure, even in protracted wounds, with excellent results and without any adverse effects or complications. Download abstract.
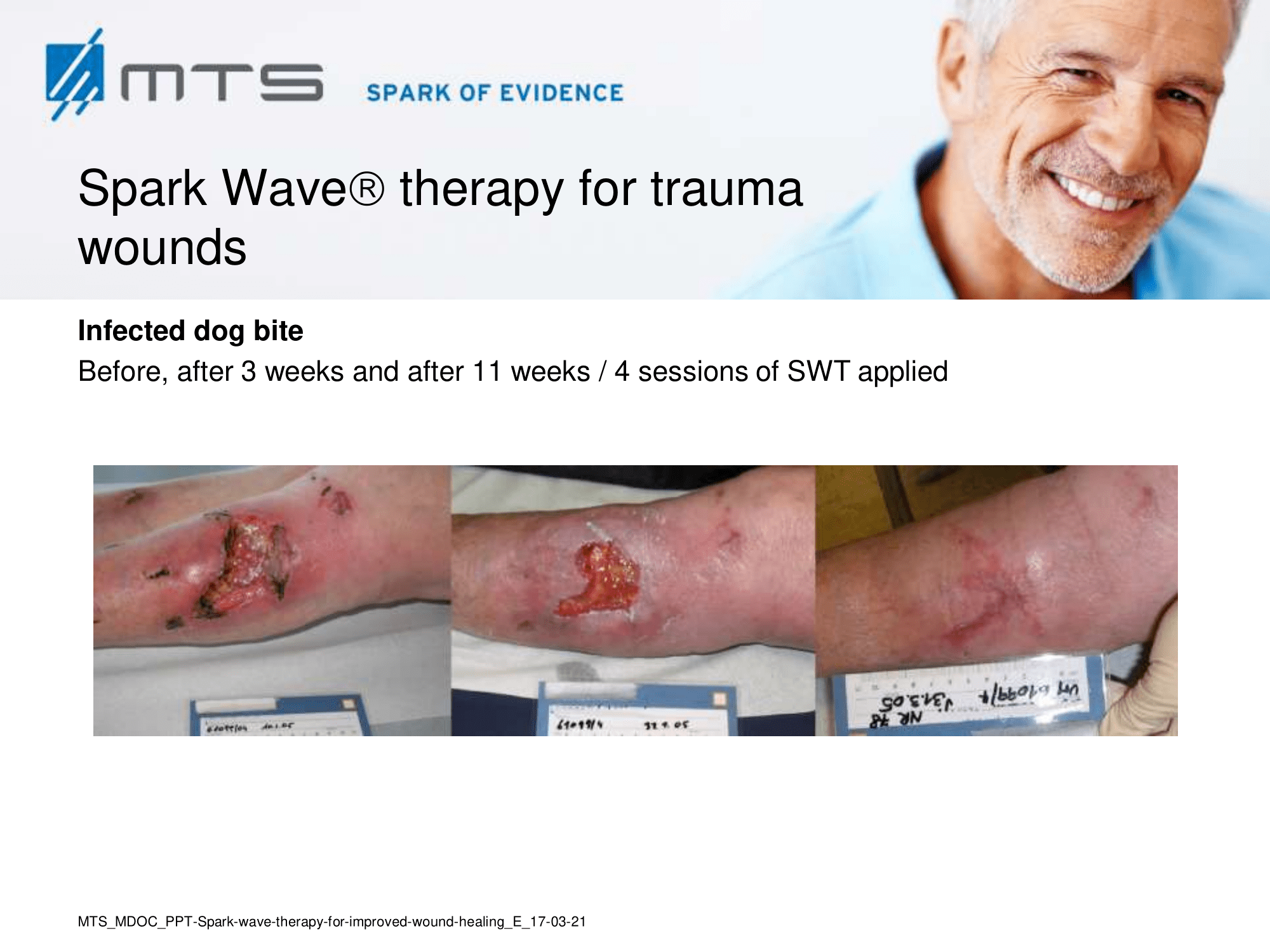
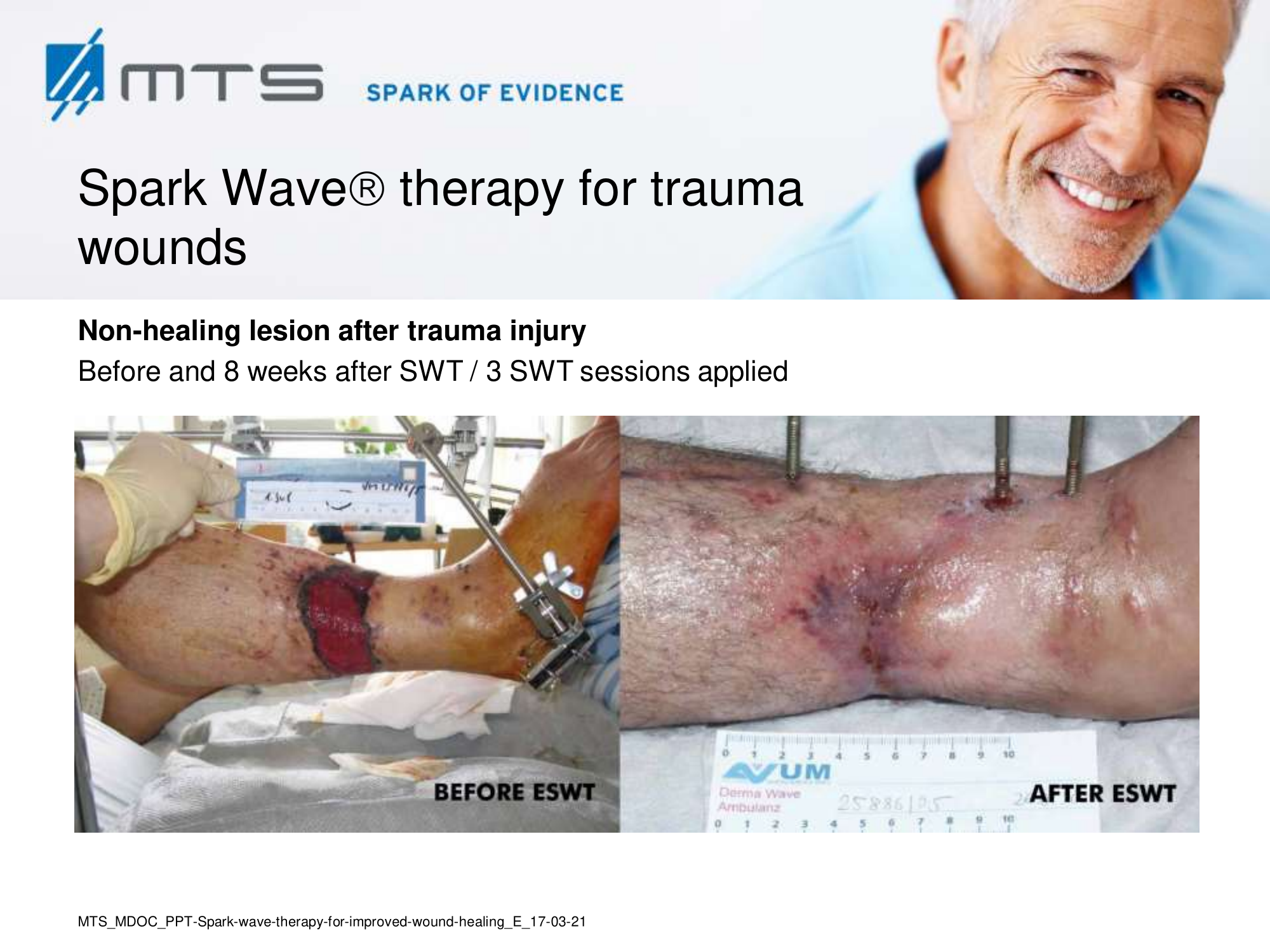
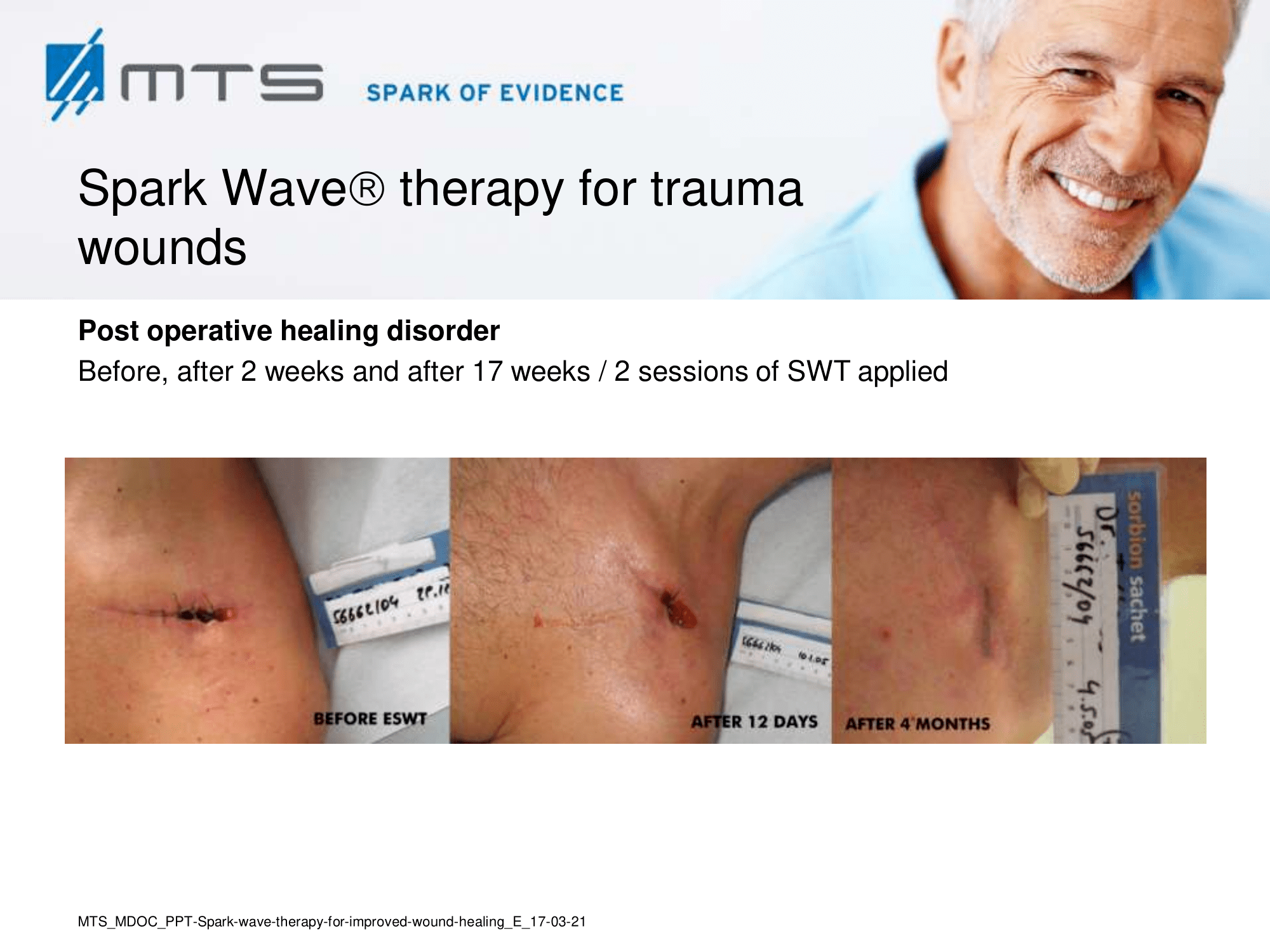
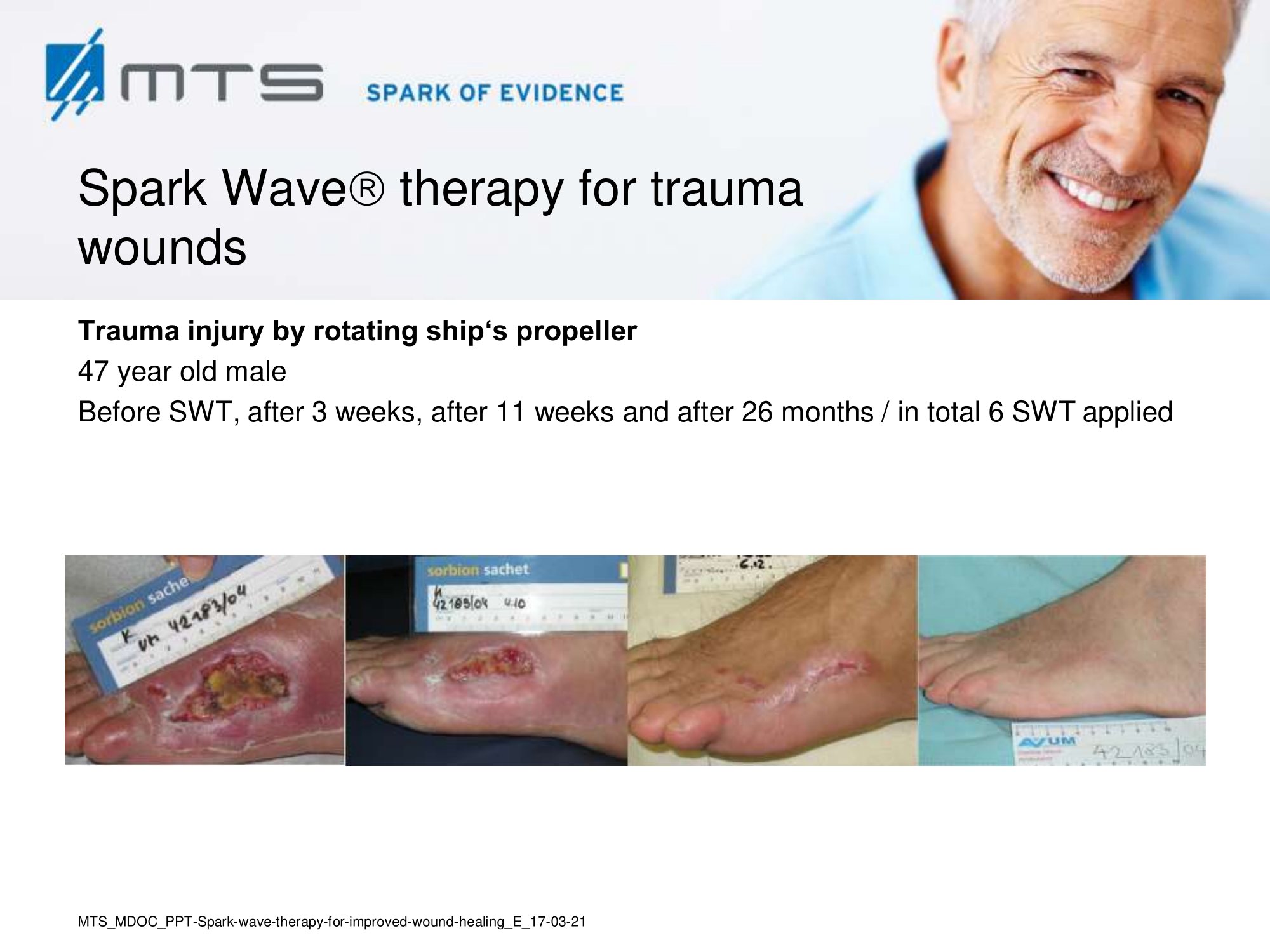
Spark Wave Therapy can certainly be used in several acute wound healing conditions like acute trauma injury and post-operative healing disorders to promote and improve healing.
On the whole, Spark Wave Therapy restores and boosts the natural self-healing of epithelial and soft tissues. It strengthens the connective tissue and evidently improves elasticity and structure. That is why ESWT is also applied in various aesthetic indications:
- Cellulite and cellulitis
- Reduction / smoothing of scar tissue
- Spider and varicose veins
- Read more about aesthetic Spark Wave indications…
-
Benefits of Spark Wave® Therapy in wound care therapy
- Can help even in „hopeless cases“ and prevent amputation
- Optimal adjunct treatment to advance conventional therapy
- Increased quality of life for patients
- Shortened period of wound treatment
- Decreased necessity of antibiotic treatment
- Practically no adverse events or complications
- Substantial supporting clinical evidence
- Reliable and cost-effective
- Noninvasive and painless
- Safe without tissue damage due to unique Spark Wave Technology
- Reliable, versatile and highly effective
-
List of MTS Spark Wave® Studies
- Prospective randomized phase II Trial of accelerated reepithelialization of superficial second-degree burn wounds using extracorporeal shock wave therapy 35
- Extracorporeal Shock Wave Therapy: An Emerging Treatment Modality for Retracting Scars of the Hands 36
- Extracorporealshockwavetherapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury 20
- Randomized control of extracorporeal shock wave therapy versus placebo for chronic decubitus ulceration 80
- Prophylactic low-energy shock wave therapy improves wound healing after vein harvesting for coronary artery bypass graft surgery: a prospective, randomized trial 121
- Shockwave therapy for systemic sclerosis 92
- Extracorporeal shockwave treatment for chronic diabetic footulcers 25
- Serum proteomic analysis of extracorporeal shock wave therapy-enhanced diabetic wound healing in a streptozotocin-induced diabetes model 87
- Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: effects of extracorporeal shock wave therapy 90
- Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes 38
- Prospective randomized trial of accelerated re-epithelization of skin graft donor sites using extracorporeal shock wave therapy 97
- Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis 29
- Angiogenic response to extracorporeal shock wave treatment in murine skin isografts 17
-
List of all references
- Dymarek, R. et al. Extracorporeal shock wave therapy as an adjunct wound treatment: a systematic review of the literature. Ostomy. Wound. Manage. 60, 26–39 (2014).
- Zhang, L., Weng, C., Zhao, Z. & Fu, X. Extracorporeal shock wave therapy for chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair and Regeneration (2017). doi:10.1111/wrr.12566
- Mittermayr, R. et al. Extracorporeal shock wave therapy (ESWT) for wound healing: Technology, mechanisms, and clinical efficacy. Wound Repair and Regeneration 20, 456–465 (2012).
- Lazarus, G. S. et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 2, 165–170 (1994).
- Snyder, D. L., Sullivan, N. & Schoelles, K. M. Skin Substitutes for Treating Chronic Wounds. Skin Substitutes for Treating Chronic Wounds (2012).
- Antonic, V., Mittermayr, R., Schaden, W. & Stojadinovic, A. Evidence supporting extracorporeal shock wave therapy for acute and chronic soft tissue wounds. Wounds a Compend. Clin. Res. Pract. 23, 204–15 (2011).
- Velnar, T., Bailey, T. & Smrkolj, V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 37, 1528–42 (2009).
- Milch, H. S., Schubert, S. Y., Hammond, S. & Spiegel, J. H. Enhancement of ischemic wound healing by inducement of local angiogenesis. Laryngoscope 120, 1744–1748 (2010).
- d’Agostino, M. C., Craig, K., Tibalt, E. & Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. International Journal of Surgery 24, 147–153 (2015).
- Ingber, D. E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827 (2006).
- Davidson, S. M., Takov, K. & Yellon, D. M. Exosomes and Cardiovascular Protection. Cardiovasc. Drugs Ther. 31, 77–86 (2017).
- Hergenreider, E. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 14, 249–256 (2012).
- Qureshi, A. A., Ross, K. M., Ogawa, R. & Orgill, D. P. Shock Wave Therapy in Wound Healing. Plast. Reconstr. Surg. 128, 721e–727e (2011).
- Sukubo, N. G., Tibalt, E., Respizzi, S., Locati, M. & d’Agostino, M. C. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int. J. Surg. 24, 124–130 (2015).
- Holfeld, J. et al. Shockwave therapy differentially stimulates endothelial cells: implications on the control of inflammation via toll-Like receptor 3. Inflammation 37, 65–70 (2014).
- Cai, Z. et al. Effects of Shock Waves on Expression of IL-6, IL-8, MCP-1, and TNF-alpha Expression by Human Periodontal Ligament Fibroblasts: An In Vitro Study. Med. Sci. Monit. 22, 914–921 (2016).
- Stojadinovic, A. et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 11, 369–380 (2008).
- Holfeld, J. et al. Low-energy shock wave treatment induces angiogenesis in ischemic muscle by stimulation of toll-like receptor 3 signaling. Eur. Hear. J. Acute Cardiovasc. Care 128, 61–62 (2013).
- Kuo, Y. R. et al. Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen. 17, 80–87 (2009).
- Davis, T. A. et al. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int Wound J 6, 11–21 (2009).
- Glaser, R. & Kiecolt-Glaser, J. K. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5, 243–251 (2005).
- Von Eiff, C. et al. Bactericidal effect of extracorporeal shock waves on Staphylococcus aureus. J. Med. Microbiol. 49, 709–712 (2000).
- Gerdesmeyer, L. et al. Antibacterial effects of extracorporeal shock waves. Ultrasound Med.Biol. 31, 115–119 (2005).
- Gollwitzer, H., Horn, C., Von Eiff, C., Henne, M. & Gerdesmeyer, L. Antibacterial effectiveness of high-energetic extracorporeal shock waves: an in vitro verification. Z. Orthop. Ihre Grenzgeb. 142, 462–466 (2004).
- Wang, C.-J. et al. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J. Surg. Res. 152, 96–103 (2009).
- Perez-Garijo, A. & Steller, H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development 142, 3253–3262 (2015).
- Zhao, Z. et al. Extracorporeal shock-wave therapy reduces progression of knee osteoarthritis in rabbits by reducing nitric oxide level and chondrocyte apoptosis. Arch. Orthop. Trauma Surg. 132, 1547–1553 (2012).
- Wang, C.-J., Wu, R.-W. & Yang, Y.-J. Treatment of diabetic foot ulcers: A comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res. Clin. Pract. 92, 187–193 (2011).
- Mittermayr, R. et al. Extracorporeal Shock Wave Therapy (ESWT) Minimizes Ischemic Tissue Necrosis Irrespective of Application Time and Promotes Tissue Revascularization by Stimulating Angiogenesis. Ann. Surg. 253, 1024–1032 (2011).
- Tonnesen, M. G., Feng, X. & Clark, R. A. F. Angiogenesis in wound healing. in Journal of Investigative Dermatology Symposium Proceedings 5, 40–46 (2000).
- Goertz, O. et al. Repetitive extracorporeal shock wave applications are superior in inducing angiogenesis after full thickness burn compared to single application. Burns 40, 1365–1374 (2014).
- Eming, S. A., Brachvogel, B., Odorisio, T. & Koch, M. Regulation of angiogenesis: Wound healing as a model. Prog. Histochem. Cytochem. 42, 115–170 (2007).
- Wang, C.-J., Ko, J.-Y., Kuo, Y.-R. & Yang, Y.-J. Molecular changes in diabetic foot ulcers. Diabetes Res. Clin. Pract. 94, 105–110 (2011).
- Fioramonti, P. et al. Extracorporeal shock wave therapy for the management of burn scars. Dermatol. Surg. 38, 778–82 (2012).
- Ottomann, C. et al. Prospective Randomized Phase II Trial of Accelerated Reepithelialization of Superficial Second-Degree Burn Wounds Using Extracorporeal Shock Wave Therapy. Ann. Surg. 255, 23–29 (2012).
- Saggini, R. et al. Extracorporeal Shock Wave Therapy: An Emerging Treatment Modality for Retracting Scars of the Hands. Ultrasound Med. Biol. 1–11 (2015). doi:10.1016/j.ultrasmedbio.2015.07.028
- Kisch, T. et al. Remote effects of extracorporeal shock wave therapy on cutaneous microcirculation. J. Tissue Viability 24, 140–145 (2015).
- Kuo, Y. R., Wang, C. T., Wang, F. S., Chiang, Y. C. & Wang, C. J. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regen. 17, 522–530 (2009).
- Kisch, T. et al. Repetitive shock wave therapy improves muscular microcirculation. J. Surg. Res. 201, 440–445 (2016).
- Tepeköylü, C. et al. Recruitment of endothelial progenitor cells in chronic hind limb ischemia by extracorporeal shock wave therapy in rats. Eur. Surg. – Acta Chir. Austriaca 43, 13 (2011).
- Zhang, X., Yan, X., Wang, C., Tang, T. & Chai, Y. The dose-effect relationship in extracorporeal shock wave therapy: the optimal parameter for extracorporeal shock wave therapy. J. Surg. Res. 186, 484–92 (2014).
- Wang, C. J., Yang, Y. J. & Huang, C. C. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatol. Int. 31, 871–877 (2011).
- Gotte, G. et al. Short-time non-enzymatic nitric oxide synthesis from L-arginine and hydrogen peroxide induced by shock waves treatment. FEBS Lett. 520, 153–155 (2002).
- Antonic, V., Mittermayr, R., Schaden, W. & Stojadinovic, A. Evidence Supporting Extracorporeal Shockwave Therapy for Acute and Chronic Soft Tissue Wounds. WOUNDS-A Compend. Clin. Res. Pract. 23, 204–215 (2011).
- Yin, T.-C., Wang, C.-J., Yang, K. D., Wang, F.-S. & Sun, Y.-C. Shockwaves enhance the osteogenetic gene expression in marrow stromal cells from hips with osteonecrosis. Chang Gung Med. J. 34, 367–74 (2011).
- Yamaya, S. et al. Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury. J. Neurosurg. 121, 1514–1525 (2014).
- Wang, L., Jiang, Y., Jiang, Z. & Han, L. Effect of low-energy extracorporeal shock wave on vascular regeneration after spinal cord injury and the recovery of motor function. Neuropsychiatr. Dis. Treat. Volume 12, 2189–2198 (2016).
- Bao, P. et al. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 153, 347–58 (2009).
- Holfeld, J. et al. Low energy shock wave therapy induces angiogenesis in acute hind-limb ischemia via VEGF receptor 2 phosphorylation. PLoS One 9, e103982 (2014).
- Wang, B. et al. Low-intensity extracorporeal shock wave therapy enhances brain-derived neurotrophic factor expression through PERK/ATF4 signaling pathway. Int. J. Mol. Sci. 18, (2017).
- Frairia, R. & Berta, L. Biological effects of extracorporeal shock waves on fibroblasts. A review. Muscles. Ligaments Tendons J. 1, 138–47 (2011).
- Hausdorf, J. et al. Stimulation of bone growth factor synthesis in human osteoblasts and fibroblasts after extracorporeal shock wave application. Arch. Orthop. Trauma Surg. 131, 303–309 (2011).
- Wang, C. J. et al. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-β1, VEGF and BMP-2 in long bone non-unions. Nitric Oxide – Biol. Chem. 20, 298–303 (2009).
- Chen, Y. J. et al. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-??1 and IGF-I expression. J. Orthop. Res. 22, 854–861 (2004).
- Wang, C.-J., Wang, F.-S. & Yang, K. D. Biological effects of extracorporeal shockwave in bone healing: a study in rabbits. Arch. Orthop. Trauma Surg. 128, 879–884 (2008).
- Raabe, O. et al. Effect of extracorporeal shock wave on proliferation and differentiation of equine adipose tissue-derived mesenchymal stem cells in vitro. Am. J. Stem Cells 2, 62–73 (2013).
- Lin, G. et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J. Sex. Med. 1–9 (2017). doi:10.1016/j.jsxm.2017.02.004
- Weihs, A. M. et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J. Biol. Chem. 289, 27090–104 (2014).
- Aschermann, I. et al. Extracorporal shock waves induce healing of chronic leg ulcers via activation of cell-cycle regulatory proteins and pro-inflammatory cytokines. Exp. Dermatol. 24, E12 (2015).
- Aschermann, I. et al. Extracorporal Shock Waves Activate Migration, Proliferation and Inflammatory Pathways in Fibroblasts and Keratinocytes, and Improve Wound Healing in an Open-Label, Single-Arm Study in Patients with Therapy-Refractory Chronic Leg Ulcers. Cell. Physiol. Biochem. 890–906 (2017). doi:10.1159/000460503
- Ramnath, D., Powell, E. E., Scholz, G. M. & Sweet, M. J. The toll-like receptor 3 pathway in homeostasis, responses to injury and wound repair. Seminars in Cell and Developmental Biology 61, 22–30 (2017).
- Tepeköylü, C. et al. Shockwaves prevent from heart failure after acute myocardial ischaemia via RNA/protein complexes. J. Cell. Mol. Med. 21, (2017).
- Cho, Y. S. et al. Effect of extracorporeal shock wave therapy on scar pain in burn patients: A prospective, randomized, single-blind, placebo-controlled study. Medicine (Baltimore). 95, e4575 (2016).
- Hausdorf, J., Schmitz, C., Averbeck, B. & Maier, M. [Molecular basis for pain mediating properties of extracorporeal shock waves]. Schmerz 18, 492–497 (2004).
- Ohtori, S. et al. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neurosci. Lett. 315, 57–60 (2001).
- Reznik, J. E., Milanese, S. & Galea, M. P. Extracorporea shock wave therapy as a treatment for neurogenic heterotopic ossification. Brain Impair. 14, 150–151 (2013).
- Fojecki, G. L., Tiessen, S. & Osther, P. J. Extracorporeal shock wave therapy (ESWT) in urology: a systematic review of outcome in Peyronie’s disease, erectile dysfunction and chronic pelvic pain. World J Urol (2016).
- Abed, J. M., McClure, S. R., Yaeger, M. J. & Evans, R. B. Immunohistochemical evaluation of substance P and calcitonin gene-related peptide in skin and periosteum after extracorporeal shock wave therapy and radial pressure wave therapy in sheep. Am. J. Vet. Res. 68, 323–328 (2007).
- Saito, S. et al. Extracorporeal Shock Wave Therapy for Digital Ulcers of Systemic Sclerosis: A Phase 2 Pilot Study. Tohoku J. Exp. Med. 238, 39–47 (2016).
- Joo SY, Cho YS, S. C. The clinical utility of extracorporeal shock wave therapy for burn pruritus: A prospective, randomized, single-blind study. Burns. [Epub ahea, (2017).
- Wang CJ, Ko JY, Chou WY1, Cheng JH, K. Y. Extracorporeal Shockwave Therapy for Treatment of Keloid Scars. Wound Repair Regen. [Epub ahea, (2018).
- Arnó, A. et al. Extracorporeal shock waves, a new non-surgical method to treat severe burns. Burns 36, 844–849 (2010).
- Meirer, R., Kamelger, F. S. & Piza-Katzer, H. Shock wave therapy: An innovative treatment method for partial thickness burns. Burns 31, 921–922 (2005).
- Cui HS, Hong AR, Kim JB, Yu JH, Cho YS, Joo SY, S. C. Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. Int J Mol Sci. (2018). doi:10.3390/ijms19010124
- Goertz, O. et al. Short-term effects of extracorporeal shock waves on microcirculation. J. Surg. Res. 194, 304–311 (2015).
- Goertz, O. et al. Extracorporeal shock waves improve angiogenesis after full thickness burn. Burns 38, 1010–1018 (2012).
- Djedovic, G., Kamelger, F. S., Jeschke, J. & Piza-Katzer, H. Effect of Extracorporeal Shock Wave Treatment on Deep Partial-Thickness Burn Injury in Rats: A Pilot Study. Plast. Surg. Int. 2014, 1–7 (2014).
- Porso, M. et al. Defocused Shock Wave Therapy for Chronic Soft Tissue Wounds in the Lower Limbs: A Pilot Study. Ultrasound Med. Biol. 43, 362–369 (2017).
- Fioramonti, P., Onesti, M. G., Fino, P., Fallico, N. & Scuderi, N. Extracorporeal shock wave therapy for the treatment of venous ulcers in the lower limbs. Ann. Ital. Chir. 83, 41–44 (2012).
- Larking, A. M., Duport, S., Clinton, M., Hardy, M. & Andrews, K. Randomized control of extracorporeal shock wave therapy versus placebo for chronic decubitus ulceration. Clin. Rehabil. 24, 222–229 (2010).
- Jeppesen, S. M., Yderstraede, K. B., Rasmussen, B. S. B., Hanna, M. & Lund, L. Extracorporeal shockwave therapy in the treatment of chronic diabetic foot ulcers: a prospective randomised trial. J. Wound Care 25, 641–649 (2016).
- Omar, M. T. A., Alghadir, A., Al-Wahhabi, K. K. & Al-Askar, A. B. Efficacy of shock wave therapy on chronic diabetic foot ulcer: A single-blinded randomized controlled clinical trial. Diabetes Res. Clin. Pract. 106, 548–554 (2014).
- Moretti, B. et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskelet. Disord. 10, 54 (2009).
- Wang, C.-J., Cheng, J.-H., Kuo, Y.-R., Schaden, W. & Mittermayr, R. Extracorporeal Shockwave Therapy in Diabetic Foot Ulcers. Int. J. Surg. 24, 207–209 (2015).
- Wang, C.-J., Wu, C.-T., Yang, Y.-J., Liu, R.-T. & Kuo, Y.-R. Long-term outcomes of extracorporeal shockwave therapy for chronic foot ulcers. J. Surg. Res. 189, 366–72 (2014).
- C.-J., W. & R.-W., W. Treatment of diabetic foot ulcers: A comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res. Clin. Pract. 92, 187–193 (2011).
- Yang, M.-Y. et al. Serum Proteomic Analysis of Extracorporeal Shock Wave Therapy–Enhanced Diabetic Wound Healing in a Streptozotocin-Induced Diabetes Model. Plast. Reconstr. Surg. 133, 59–68 (2014).
- Hayashi, D. et al. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: A critical role of endothelial nitric oxide synthase. Wound Repair Regen. 20, 887–895 (2012).
- Yang, G., Luo, C., Yan, X., Cheng, L. & Chai, Y. Extracorporeal Shock Wave Treatment Improves Incisional Wound Healing in Diabetic Rats. Tohoku J. Exp. Med. 225, 285–292 (2011).
- Zins, S. R., Amare, M. F., Tadaki, D. K., Elster, E. A. & Davis, T. A. Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: Effects of extracorporeal shock wave therapy. Angiogenesis 13, 293–304 (2010).
- Yan, X. et al. [Effect of extracorporeal shock wave therapy on diabetic chronic wound healing and its histological features]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26, 961–967 (2012).
- Belloli, L. et al. Shock wave therapy for systemic sclerosis. Rheumatology International 33, 1099–1100 (2013).
- Blumhardt, S. et al. Safety and efficacy of extracorporeal shock wave therapy (ESWT) in calcinosis cutis associated with systemic sclerosis. Clin. Exp. Rheumatol. 34, 177–180 (2016).
- Sultan-Bichat, N. et al. Treatment of calcinosis cutis by extracorporeal shock-wave lithotripsy. J. Am. Acad. Dermatol. 66, 424–429 (2012).
- Tinazzi, E. et al. Effects of shock wave therapy in the skin of patients with progressive systemic sclerosis: A pilot study. Rheumatol. Int. 31, 651–656 (2011).
- Chan, A. Y. K. & Li, E. Electric shock wave lithotripsy (ESWL) as a pain control measure in dermatomyositis with calcinosis cutis-old method, new discovery. Clin. Rheumatol. 24, 172–3 (2005).
- Ottomann, C. et al. Prospective randomized trial of accelerated re-epithelization of skin graft donor sites using extracorporeal shock wave therapy. J Am Coll Surg 211, 361–367 (2010).
- Birgin E, Gebhardt C, Hetjens S, Fischer S, Rückert F, R. M. Extracorporal Shock Wave Therapy Enhances Receptor for Advanced Glycated End-Product-Dependent Flap Survival and Angiogenesis. Ann Plast Surg [Epub ahea, (2018).
- Zhang, X. et al. The effect of autologous endothelial progenitor cell transplantation combined with extracorporeal shock-wave therapy on ischemic skin flaps in rats. Cytotherapy 16, 1098–1109 (2014).
- Reichenberger, M. A. et al. Extracorporeal shock wave treatment protects skin flaps against ischemia-reperfusion injury. Injury 43, 374–380 (2012).
- Keil, H. et al. Preoperative shock wave treatment enhances ischemic tissue survival, blood flow and angiogenesis in a rat skin flap model. Int. J. Surg. 9, 292–296 (2011).
- Reichenberger, M. A. et al. Optimal Timing of Extracorporeal Shock Wave Treatment to Protect Ischemic Tissue. Ann. Plast. Surg. 67, 539–544 (2011).
- Reichenberger, M. A. et al. Comparison of extracorporal shock wave pretreatment to classic surgical delay in a random pattern skin flap model. Plast. Reconstr. Surg. 127, 1830–7 (2011).
- Kamelger, F., Oehlbauer, M., Piza-Katzer, H. & Meirer, R. Extracorporeal shock wave treatment in ischemic tissues: What is the appropriate number of shock wave impulses? J. Reconstr. Microsurg. 26, 117–121 (2010).
- Reichenberger, M. a, Germann, G., Roth, H. J., Meirer, R. & Engel, H. Preoperative shock wave therapy reduces ischemic necrosis in an epigastric skin flap model. Ann. Plast. Surg. 63, 682–4 (2009).
- Yan, X., Zeng, B., Chai, Y., Luo, C. & Li, X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann. Plast. Surg. 61, 646–653 (2008).
- Kuo, Y. R. et al. Extracorporeal Shock Wave Enhanced Extended Skin Flap Tissue Survival via Increase of Topical Blood Perfusion and Associated with Suppression of Tissue Pro-Inflammation. J. Surg. Res. 143, 385–392 (2007).
- Meirer, R. et al. Comparison of the effectiveness of gene therapy with vascular endothelial growth factor or shock wave therapy to reduce ischaemic necrosis in an epigastric skin flap model in rats. J. Plast. Reconstr. Aesthet. Surg. 60, 266–71 (2007).
- Meirer, R. et al. Shock wave therapy reduces necrotic flap zones and induces VEGF expression in animal epigastric skin flap model. J. Reconstr. Microsurg. 23, 231–235 (2007).
- Meirer, R., Kamelger, F. S., Huemer, G. M., Wanner, S. & Piza-Katzer, H. Extracorporal shock wave may enhance skin flap survival in an animal model. Br. J. Plast. Surg. 58, 53–57 (2005).
- Huemer, G. M. et al. Comparison of the effectiveness of gene therapy with transforming growth factor-beta or extracorporal shock wave therapy to reduce ischemic necrosis in an epigastric skin flap model in rats. Wound Repair Regen. 13, 262–268 (2005).
- Knobloch, K. & Vogt, P. M. High-energy focussed extracorporeal shockwave therapy reduces pain in plantar fibromatosis (Ledderhose’s disease). BMC Res. Notes 5, (2012).
- Chen, P. C. et al. Noninvasive Shock Wave Treatment for Capsular Contractures After Breast Augmentation: A Rabbit Study. Aesthetic Plast. Surg. 40, 435–445 (2016).
- Fischer, S. et al. Multiple extracorporeal shock wave therapy degrades capsular fibrosis after insertion of silicone implants. Ultrasound Med. Biol. 41, 781–789 (2015).
- Wu, Y. C. et al. Preliminary study of non-invasive shock wave treatment of capsular contracture after breast implant: Animal model. in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 1108–1111 (2013). doi:10.1109/EMBC.2013.6609699
- Bae, H. & Kim, H. J. Clinical outcomes of extracorporeal shock wave therapy in patients with secondary lymphedema: A pilot study. Ann. Rehabil. Med. 37, 229–234 (2013).
- Cebicci, M. A. et al. Extracorporeal Shock Wave Therapy for Breast Cancer–Related Lymphedema: A Pilot Study. Arch. Phys. Med. Rehabil. 97, 1520–1525 (2016).
- Kim, S.-Y., Bae, H. & Ji, H. M. Computed Tomography as an Objective Measurement Tool for Secondary Lymphedema Treated With Extracorporeal Shock Wave Therapy. Ann. Rehabil. Med. 39, 488 (2015).
- Kubo, M. et al. Extracorporeal shock wave therapy ameliorates secondary lymphedema by promoting lymphangiogenesis. J. Vasc. Surg. 52, 429–434 (2010).
- Serizawa, F. et al. Extracorporeal shock wave therapy induces therapeutic lymphangiogenesis in a rat model of secondary lymphoedema. Eur. J. Vasc. Endovasc. Surg. 42, 254–60 (2011).
- Dumfarth, J. et al. Prophylactic Low-Energy Shock Wave Therapy Improves Wound Healing After Vein Harvesting for Coronary Artery Bypass Graft Surgery: A Prospective, Randomized Trial. Ann. Thorac. Surg. 86, 1909–1913 (2008).